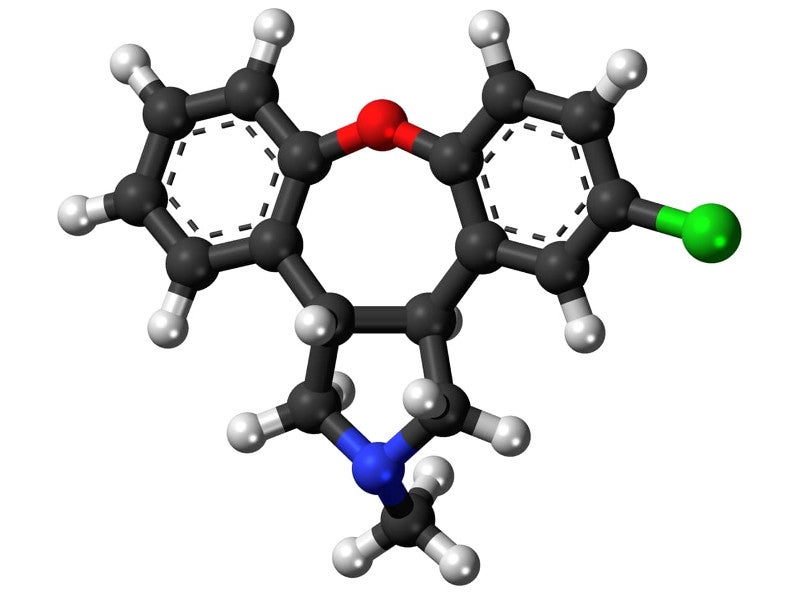
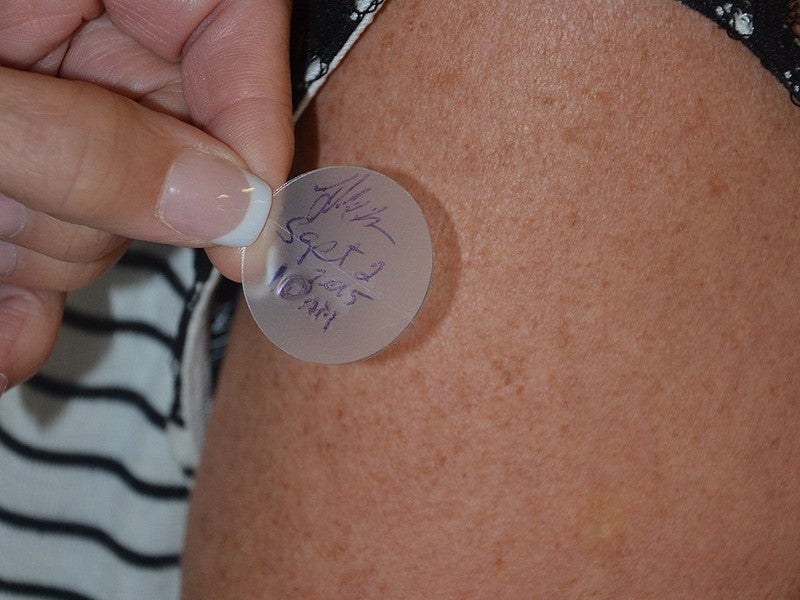
Secuado® (asenapine) is an atypical antipsychotic drug approved for the treatment of schizophrenia in adult patients. It is the first drug approved as transdermal patch formulation for schizophrenia treatment in the US.
Secuado was developed by Hisamitsu Pharmaceutical, a company based in Japan, along with its US subsidiary, Noven Pharmaceuticals. Hisamitsu’s Transdermal Drug Delivery System (TDDS) technology was used to develop the drug.
The company submitted a new drug application (NDA) for the drug to the US Food and Drug Administration (FDA) in December 2018, while the FDA approval was received in October 2019.
Asenapine was first developed by Allergan in the form of sublingual tablets for the treatment of schizophrenia and approved in August 2009 under the brand name Saphris®.
Secuado is available as a translucent rounded square transdermal patch with a release liner applied on the skin. It comes in three dose strengths, 3.8mg / 24 hours, 5.7mg / 24 hours and 7.6mg / 24 hours.
Schizophrenia causes and symptoms
Schizophrenia is a chronic psychological disorder that impacts an individual’s thinking ability and causes behavioural disorders. The disease is characterised by loss of the sense of reality in patients, hallucinations and delusions, which typically start between the ages of 16 and 30 years.
Some of the other common symptoms of the disease are reduced facial expressions or expression of emotions, social withdrawal, disorganised speech, reduced working memory, lack of motivation and impaired sustained attention.
Several factors are believed to increase the risk of schizophrenia, such as genetic factors, environmental factors including exposure to virus, or malnutrition before birth, as well as a structural and chemical change in the brain. An estimated 1% of people worldwide are affected by schizophrenia.
Secuado’s mechanism of action
The exact mechanism of Secuado for treating schizophrenia is not well known. The drug is believed to impart antagonist activity at the dopamine (D2) and 5-HT2A receptors.
Clinical studies on Secuado
The clinical efficacy of asenapine was determined based on clinical studies performed with its sublingual formulation on patients with schizophrenia.
The FDA’s approval for the asepanine transdermal system (formerly HP-3070) comes from a six-week randomised, fixed-dose, placebo-controlled phase three clinical study. The double-blind study enrolled 617 schizophrenia patients.
The primary endpoint of the study was the Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impressions-Severity (CGI-S) rating scores at week six compared to placebo. The scores measure the psychiatric signs and symptoms in the patients.
PANSS measures the positive and negative symptoms of schizophrenia and general psychopathology of patients on a 30-point scale, while CGI-S measures a patient’s current state of the disease and overall clinical state on a one to seven-point scale.
The patients received either fixed doses of 3.8mg Secuado or 7.6mg Secuado in every 24 hours or placebo for six weeks. At week six, both the doses of Secuado were found to be significantly superior to placebo.
The change in PANSS total score from baseline was -22.1 in patients receiving 3.8mg Secuado, -20.4 in patients receiving 7.6mg Secuado, -15.5 in patients receiving the placebo. The negative value represents an improvement.
Some of the most common adverse events observed in patients during the clinical study were an extrapyramidal disorder, reaction at the application site and increase in weight.
Clinical study on transdermal system adhesion
The transdermal system adhesion was tested in a clinical study that enrolled 40 patients.
The subjects wore one 3.8mg per 24 hours Sequado system and all of them exhibited at least 75% surface area adhesion throughout the 24 hours.
In another study, out of 39 patients that wore one 7.6mg Secuado for 24 hours, 92% showed 75% or more surface area adhesion throughout the period.