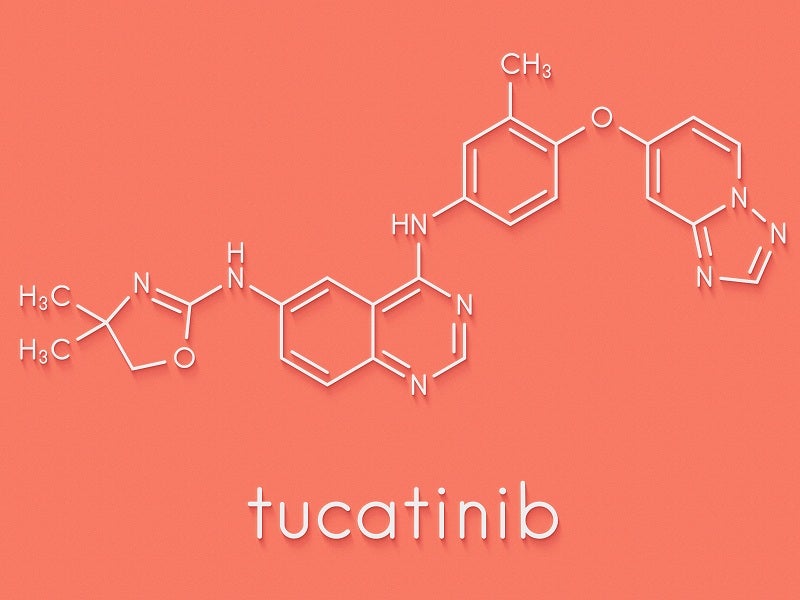
TUKYSA™ (tucatinib) is the first HER-2 tyrosine kinase inhibitor approved in combination with trastuzumab and capecitabine for the treatment of HER2-positive metastatic breast cancer.
Developed by Seattle Genetics, TUKYSA (tucatinib) was approved by the US Food and Drug Administration (FDA) in collaboration with Health Canada, Australian Therapeutic Goods Administration (TGA), Swissmedic (SMC, Switzerland) and Health Sciences Authority (HSA, Singapore) in April 2020.
The new drug application (NDA) for TUKYSA (tucatinib) was submitted to the FDA in December 2019 under the real-time oncology review (RTOR) pilot programme.
FDA granted breakthrough therapy designation for TUKYSA (tucatinib) in December 2019. The drug also received fast track designation and orphan drug designation from the FDA.
European Medicines Agency (EMA) validated the marketing authorisation application (MAA) for TUKYSA (tucatinib) in combination with trastuzumab and capecitabine for the treatment of adult patients with locally advanced unresectable or metastatic HER2-positive breast cancer in January 2020.
FDA accepted the priority review for TUKYSA (tucatinib) in February 2020; its Prescription Drug User Fee Act (PDUFA) target action date is set as 20 August 2020.
TUKYSA (tucatinib) is available as a yellow-coloured tablet with a recommended dose of 200mg administered orally twice daily.
HER2-positive breast cancer causes and symptoms
Breast tumours with increased levels of human epidermal growth factor receptor 2 (HER2) proteins are categorised as HER2-positive breast cancer.
HER2 proteins are responsible for the growth of the cancerous cells present in the breast. These cancerous cells grow and spread faster than other breast cancers.
The HER2 gene produces HER2 proteins, also referred to as HER2/neu proteins. HER2 are the receptor proteins found in the external surface of breast cells. In typical conditions, HER2 receptor controls the growth of breast cells.
HER2 gene, however, creates multiple copies of itself, causing gene amplification in 10-20% of the breast cancers. This leads to the production of a large number of copies of HER2 proteins or receptors, known as HER2 protein overexpression. The condition causes breast cell growth and multiplication in an uncontrolled manner.
Typical symptoms include changes in breast shape, swelling of the breast, pain in breast or nipple, dimpling or skin irritation, discharge from the breast, redness and increased thickness of breast skin or nipple.
Tucatinib mechanism of action
Tucatinib is an inhibitor of HER2 tyrosine kinase. It inhibits the growth of HER2-expressing tumour proteins in vivo and in vitro. TUKYSA suppresses the phosphorylation of HER2 and HER3 proteins, leading to inhibition of downstream AKT and MAPK signalling and growth of the cells.
TUKYSA also exhibits anti-tumour activity in HER2 expressing tumour cells. The drug in combination with the anti-HER2 antibody trastuzumab showed increased activity of anti-tumour both in vivo and in vitro when compared to either drug alone.
Clinical trials on TUKYSA
FDA approval of TUKYSA in combination with trastuzumab and capecitabine was based on the double-blind and a placebo-controlled HER2CLIMB clinical trial.
HER2CLIMB trial enrolled 612 patients with HER2-positive, unresectable locally advanced or metastatic breast cancer, with or without brain metastases. Patients were previously treated with either trastuzumab/pertuzumab or ado-trastuzumab emtansine (T-DM1) and a combination of these drugs.
Patients were randomised (2:1) to receive 300mg of TUKYSA or oral placebo twice daily with 8mg/kg of trastuzumab as a loading dose on day 1 of cycle 1 and then a maintenance dose of 6mg/kg on day 1 of every 21-day cycle. Capecitabine 1000mg/m2 was given orally twice daily on day 1 through 14 of every 21-day cycle.
An additional alternated dosing regimen of trastuzumab 600mg was administered subcutaneously on Day 1 of every 21-day cycle. Patients were treated until disease progression or unacceptable toxicity.
The primary endpoint was progression-free survival (PFS), or the time duration when there was no tumour growth estimated by blinded independent central review (BICR) in the first 480 randomised patients.
Other additional endpoints were overall survival (OS), PFS among patients with a history or presence of brain metastases, and confirmed objective response rate (ORR).
Patients treated with TUKYSA in combination with trastuzumab and capecitabine witnessed a 56% reduction in PFS or cancer progression when compared to patients who received trastuzumab and capecitabine alone.
The treatment with TUKYSA decreased the risk of death by 32% when compared to trastuzumab and capecitabine alone. Administered in patients with brain metastases, The drug reduced PFS by 53.5% versus trastuzumab and capecitabine alone.
Patients who received TUKYSA in combination with trastuzumab and capecitabine had a confirmed objective response rate (ORR) of 40.6% while those who received trastuzumab and capecitabine alone had an ORR of 22.8%.
The most common adverse reactions occurred in more than 20% of the patients who received TUKYSA were nausea, fatigue, diarrhoea, hepatotoxicity, palmar-plantar erythrodysesthesia and vomiting, as well as abdominal pain, rash, inflammation of the mouth and lips, headache, anaemia and decreased appetite.