Retevmo™ (selpercatinib) is the first therapy approved for the treatment of three rearranged during transfection (RET)-driven cancer indications.
Retevmo (selpercatinib) is indicated for metastatic RET fusion-positive non-small cell lung cancer (NSCLC), advanced or metastatic RET-mutant medullary thyroid cancer (MTC) and advanced or metastatic RET fusion-positive thyroid cancer.
Retevmo approvals
Developed by Loxo Oncology, a subsidiary of Eli Lilly and Company, Retevmo (selpercatinib) received approval from the US Food and Drug Administration (FDA) under accelerated approval regulations in May 2020.
Retevmo (selpercatinib) was also granted orphan drug, breakthrough therapy and priority review designations by the FDA.
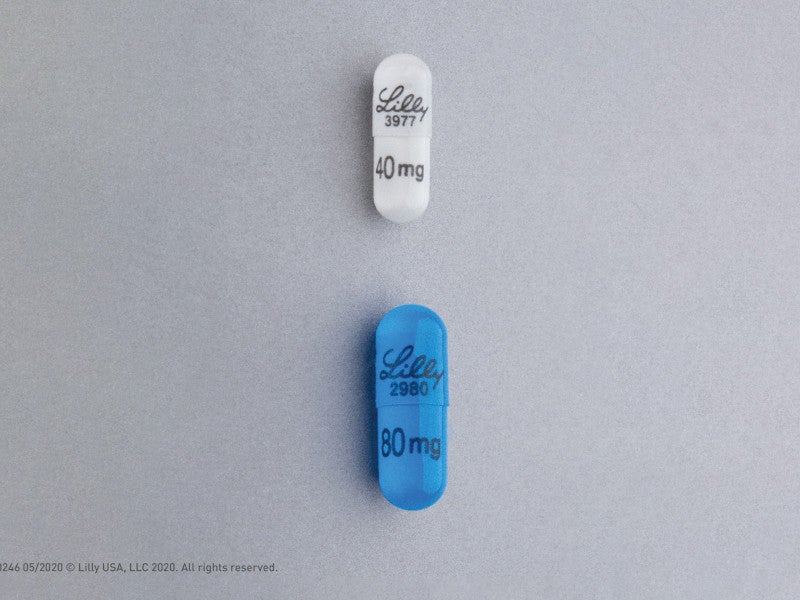
The drug is available as an oral capsule with a recommended dose of 120mg or 160mg based on the weight of patients, administered twice a day.
RET-driven cancers causes and symptoms
The RET gene can drive metastatic thyroid cancer and metastatic NSCLC in a person’s body.
Two prominent types of the cancer-promoting RET genomic alterations are activating point mutations and fusions, which lead to hyperactive signalling and uncontrolled cell proliferation.
RET activation point mutations account for approximately 90% of familial medullary thyroid cancer (MTC) and 60% of sporadic MTC.
MTC develops in the thyroid gland (C-cells), which produces calcitonin, a hormone that controls the amount of calcium in the blood.
RET fusions are found in approximately 10-20% of papillary thyroid cancers (PTC) and undifferentiated thyroid cancers, as well as 2% of non-small cell lung cancers.
The most common signs and symptoms of lung cancer are coughing up blood, appetite loss, chest pain, hoarseness, fatigue, weakness, weight loss, bronchitis, pneumonia, wheezing and shortness of breath.
Symptoms of thyroid cancers include swelling of glands in the neck, a lump near Adam’s apple, dysphagia, dyspnea, throat or neck pain, and a persistent cough not caused by a cold.
Selpercatinib mechanism of action
Selpercatinib is a potent and selective RET kinase inhibitor. The drug can affect both tumour cells as well as healthy cells, resulting in side effects with some of them exhibiting serious consequences.
Retevmo is a targeted therapy that targets the specific driver of certain RET-driven advanced or metastatic cancers. RET alterations in humans cause cells to multiply abnormally, creating a potentially cancerous tumour. Retevmo acts as an inhibitor that controls the RET kinase enzyme and prevents the growth of cancerous cells.
Selpercatinib blocks the multiple mutated RET isoforms and wild-type RET, as well as VEGFR1 and VEGFR3.
It demonstrated anti-tumour activity in mice model of a RET fusion-positive patient-derived tumour intracranially implanted into the brain.
The two confirmatory phase 3 trials (LIBRETTO-431 and LIBRETTO-531) for Retevmo are currently enrolling patients.
Clinical trials on Retevmo
FDA approval of Retevmo (LOXO-292) was based on the single-arm, open-label, multi-cohort, multicentre, Phase 1/2 LIBRETTO-001 clinical trial.
Patients with RET fusion-positive NSCLC (n=144), RET-mutant MTC (n=143), RET fusion-positive thyroid cancer (n=27) and other solid tumours with RET alterations enrolled in the study.
A dose of 160mg Retevmo was given orally to adult patients twice a day until the progression of the disease showed unacceptable levels of toxicity.
The primary measure outcome of the trial was overall response rate (ORR) and duration of response (DOR), as determined according to RECIST v1.1 by a blinded independent review committee (BIRC).
ORR was 64%, and the median DOR lasted at least six months for RET fusion-positive NSCLC patients previously treated with platinum chemotherapy. ORR was 84%, and DOR lasted at least six months for patients who had never undergone treatment.
Meanwhile, the ORR was 69%, and the median DOR lasted at least six months for patients with advanced or metastatic RET-mutant MTC who were previously treated with cabozantinib, vandetanib or both.
ORR was 73%, while the DOR lasted at least six months for patients with advanced or metastatic RET-mutant MTC previously not treated with cabozantinib or vandetanib.
The ORR was 79%, while the duration of response was at least six months for the patients with RET fusion-positive thyroid cancers who were radioactive iodine-refractory (RAI, if an appropriate treatment option) and had received another prior systemic treatment.
Furthermore, ORR was 100% and DOR lasted at least six months for patients who had not received therapy except RAI.
The most common adverse reactions observed during the trial were dry mouth, hypertension, diarrhoea, fatigue, increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzymes in the liver and low blood platelet count.
Patients also experienced rash, swelling in the body or limbs, increased blood sugar, decreased platelets and leukocytes, increased cholesterol, alkaline phosphatase and creatinine, decreased albumin, sodium and calcium, as well as constipation and pneumonia.