Yescarta™ (axicabtagene ciloleucel) is one of the first chimeric antigen receptor T-cell (CAR T) therapies indicated for the treatment of relapsed / refractory large B-cell lymphoma in adult patients.
The drug is administered to patients that were previously treated with two or more lines of systemic therapy. It is not meant for patients with primary central nervous system (CNS) lymphoma.
Yescarta is manufactured and distributed by Kite Pharma, a subsidiary of US-based biopharmaceutical company Gilead Sciences.
Kite Pharma submitted a biologics license application (BLA) for Yescarta in March 2017. It received approval from the US Food and Drug Administration (FDA) as the second CAR-T therapy, in October 2017. The drug also received breakthrough therapy orphan designations from the FDA.
The company initiated its CAR T clinical programme in the EU in August 2017. The European Medicines Agency (EMA) approved the drug in August 2018 for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) in adult patients. Health Canada approved the drug in February 2019.
Kite Pharma and Fosun Pharma formed a joint venture (JV) named FosunKite in April 2017 for the development, manufacturing and commercialisation of the drug in China. The JV also holds the option for additional products including Kite’s two T-cell receptor product candidates.
B-cell lymphoma disease details
B-cell lymphoma is one of the two main types of non-Hodgkin lymphoma (NHL), a cancer that develops from white blood cells. Symptoms include night sweats, fever, weight loss and enlarged lymph nodes.
B-cell lymphoma is divided into low and high grade. It affects the B cells, which help protect the body against bacteria and infections. The types of NHL include diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma and primary mediastinal B-cell lymphoma.
NHLs develop most commonly in immunocompromised individuals and in older adults. An estimated 72,000 new cases in the US are diagnosed a year.
Yescarta’s mechanism of action
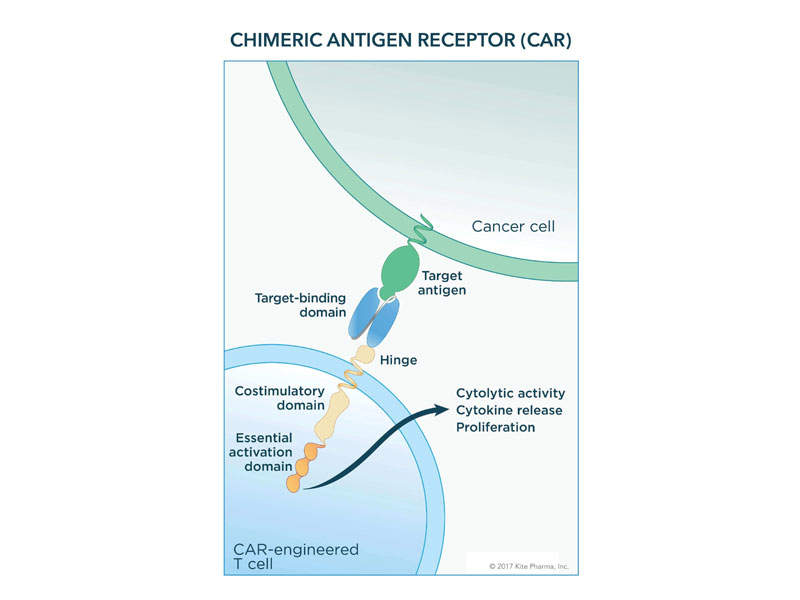
The patient’s T-cells are removed and grown in the laboratory through ex-vivo gene modification to generate a CAR consisting of anti-CD19 CAR T-cells linked to CD28 and CD3-zeta co-stimulatory domains. The anti-CD19 CAR T-cells are then transferred back to the patient to recognise and eliminate CD19-expressing target cells.
The engagement of anti-CD19 CAR T-cells and CD19-expressing target cells allows the CD28 and CD3-zeta co-stimulatory domains to activate downstream signalling cascades.
This process will lead to the activation of T-cells, proliferation, acquisition of effector functions, and release of inflammatory cytokines and cytokines, as well as killing CD19-expressing cells.
The drug is available in a 68ml suspension per infusion bag and is packed in a metal cassette. It can be administered as a suspension of 2 × 106 CAR-positive viable T-cells per kg body weight, with a total dose of 2 × 108 CAR-positive viable T-cells.
Clinical trials on Yescarta
The FDA’s approval of Yescarta was based on results obtained from ZUMA-1, which was a pivotal national clinical trial that ran for more than two years.
The single-arm, open-label, multi-centre study enrolled 111 adult patients with large B-cell non-Hodgkin lymphoma who had previously received two treatments. Of the 111 patients, 101 were administered with single CAR-T infusion.
Results of the ZUMA-1 study indicated that the axicabtagene ciloleucel therapy produced an improved objective response rate in 72% of patients with a median time-to-response of 0.9 months. There was also a complete remission rate of 51% for patients treated with axicabtagene ciloleucel.
Yescarta caused higher adverse reactions such as fever, infections, febrile neutropenia, hypotension, cytokine release syndrome (CRS) and hypoxia in approximately 13% of the patients, while 52% experienced serious adverse reactions and 31% neurologic toxicities.
Fatal cases of CRS also occurred during the study.