Tepmetko (tepotinib) is an oral kinase inhibitor approved for the treatment of patients with metastatic non-small cell lung cancer (NSCLC). It is the first and only US Food and Drug Administration (FDA) approved mesenchymal-epithelial transition (MET) inhibitor with once-daily oral dosing.
Discovered and developed by Germany-based Merck, tepotinib is available as oval, white-pink, biconvex film-coated tablets of a 225mg dosage strength. The tablet is embossed with the letter M on one side.
NSCLC causes and symptoms
Lung cancer is the most common type of cancer throughout the world, with more than two million new cases a year. In 2020, the US recorded around 228,000 new cases of lung cancer and more than 135,000 mortalities due to lung cancer.
Some of the most common symptoms of NSCLC include the worsening of chronic cough, cough producing blood, wheezing, unexplained weight loss, chest pain or painful breathing, decreased appetite, and lung infections such as bronchitis and pneumonia. Advanced stage symptoms include bone pain, headaches or seizures due to brain metastases, and lumps in lymph nodes.
While the exact underlying cause of NSCLC is unknown, risk factors include smoking, passive smoking, radiation therapy for other cancers, exposure to radon and asbestos, arsenic in drinking water, genetics and air pollution.
Around 85% of lung cancers are NSCLC, while MET exon 14 (METex14) skipping alterations are found in 3% to 5% of NSCLC cases. NSCLC is classified into three types, namely adenocarcinoma, squamous cell carcinoma and large cell carcinoma.
Tepmetko approvals
In June 2020, a new drug application (NDA) for tepotinib as a treatment for metastatic non-small cell lung cancer was submitted to the FDA. In August 2020, the application was accepted and granted for priority review.
In February 2021, the FDA granted accelerated approval to Tepmetko (tepotinib) for the treatment of adult patients with metastatic non-small cell lung cancer harbouring METex14-skipping alterations. It also granted breakthrough therapy designation (BTD) and orphan drug designation (ODD) to tepotinib.
In March 2020, the Japanese Ministry of Health, Labour and Welfare (MHLW) approved tepotinib for the treatment of patients with advanced NSCLC with METex14-skipping alterations. The MHLW also granted Sakigake fast-track designation and ODD to the drug.
In November 2020, the European Medicines Agency (EMA) validated Merck’s marketing authorisation application for reviewing tepotinib as a treatment for advanced NSCLC with METex14 skipping alterations in adult patients.
Tepotinib mechanism of action
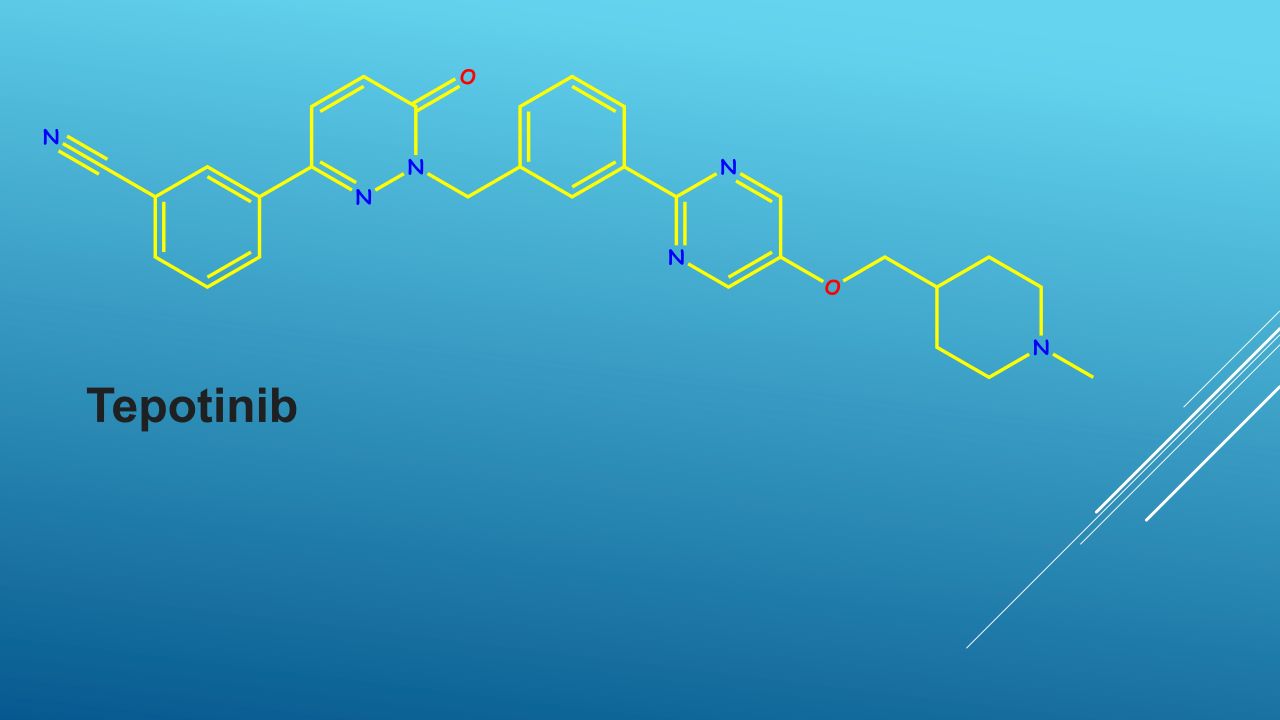
Tepotinib is a kinase inhibitor directed against MET, including METex14-skipping alterations.
Administered once daily, tepotinib inhibits MET phosphorylation and subsequent downstream signalling pathways to inhibit tumour growth, anchorage-independent growth and the migration of MET-dependent tumour cells. It has also been shown to inhibit melatonin 2 and imidazoline 1 receptors at clinically relevant concentrations.
Clinical trials on Tepmetko
The FDA’s approval of tepotinib was based on the outcome of the pivotal Phase II VISION clinical trial. The multi-centre, non-randomised, single-arm, open-label, multi-cohort clinical trial enrolled 152 patients with advanced or metastatic NSCLC with METex14-skipping alterations. Of the patients in the trial, 83 had previously been treated while 69 were treatment-naive.
Participants in the clinical study were administered 450mg of tepotinib orally once a day until their disease progressed or they faced unacceptable toxicity. The primary efficacy outcome measure of the study was the overall response rate (ORR), as assessed by a blinded independent review committee (BIRC), while the secondary outcome measure was the duration of response (DOR).
The ORR was 43% among the treatment-naive patients, with a median DOR of 10.8 months. The ORR was also 43% among the previously treated patients, with a median DOR of 11.1 months.
The most common adverse reactions observed in patients who received Tepmetko during the clinical trial included edema, nausea, fatigue, musculoskeletal pain, diarrhoea and dyspnoea.