KERENDIA® (finerenone) is a first-in-class, nonsteroidal mineralocorticoid receptor antagonist (MRA) indicated to reduce the progression of chronic kidney disease, risk of kidney failure and risk of cardiovascular disease in adult patients with chronic kidney disease (CKD) associated with type 2 diabetes (T2D).
The drug reduces several cardiovascular risks, including non-fatal myocardial infarction (MI), hospitalisation for heart failure and cardiovascular death in adult patients with CKD associated with T2D.
Developed by German multinational pharmaceutical company Bayer, KERENDIA is available as film-coated, oblong tablets in two strengths, including pink-coloured 10mg tablets and yellow-coloured 20mg tablets.
Regulatory approvals for KERENDIA
Bayer submitted a new drug application (NDA) to the US Food and Drug Administration (FDA) for KERENDIA (finerenone) in November 2020. The NDA was accepted and the drug was granted priority review for patients with CKD and T2D in January 2021. The drug also holds fast-track designation for the condition.
The FDA approved KERENDIA in July 2021, while the European Union (EU) approved the drug for CKD at third and fourth stages with albuminuria associated with T2D in adults in February 2022. The EU further approved KERENDIA to treat early stages of CKD associated with T2D in February 2023.
The Japanese Ministry of Health, Labour and Welfare (MHLW) approved KERENDIA in March 2022 to treat early to late-stage CKD and T2D based on the positive outcomes of FIDELITY, a prespecified pooled evaluation of the Phase III FIDELIO-DKD and FIGARO-DKD clinical studies.
The Chinese National Medical Products Administration approved the drug initially for the treatment of late-stage CKD associated with T2D in adults in June 2022. The label was further expanded to include earlier stages of CKD associated with T2D and reduce the patient’s risk of cardiovascular outcomes in May 2023.
KERENDIA was approved in Saudi Arabia to reduce the risk of sustained estimated glomerular filtration rate (eGFR) decline, end-stage kidney disease, cardiovascular death, nonfatal MI and hospitalisation for heart failure in adult patients with CKD associated with T2D in August 2022.
In October 2022, the drug was approved in Canada as an adjunct to standard of care therapy in adults with chronic CKD and T2D to reduce the risk of end-stage kidney disease and a sustained decrease in eGFR, cardiovascular death, non-fatal MI and hospitalisation for heart failure.
The study demonstrated that KERENDIA reduced the risk of CV events in a broad range of patients with stages one to four CKD and T2D.
Chronic kidney disease causes and symptoms
CKD is a gradual loss of kidney function leading to renal failure. It typically progresses silently without any symptoms in the early stages. The disease is often associated with T2D and about 40% of the T2D patients develop CKD.
The condition develops when the kidneys are damaged and become unable to filter blood normally. Patients may experience problems associated with fluid, electrolytes and waste build-up due to improper filtration and the disease can increase the risk of developing heart disease. Diabetes is currently the primary cause of CKD and kidney failure in the US.
Symptoms associated with CKD are fatigue, loss of appetite, weight loss, swollen ankles, feet or hands, tiredness, itchy skin, muscle cramps, headache, insomnia and blood in the urine.
Finerenone’s mechanism of action
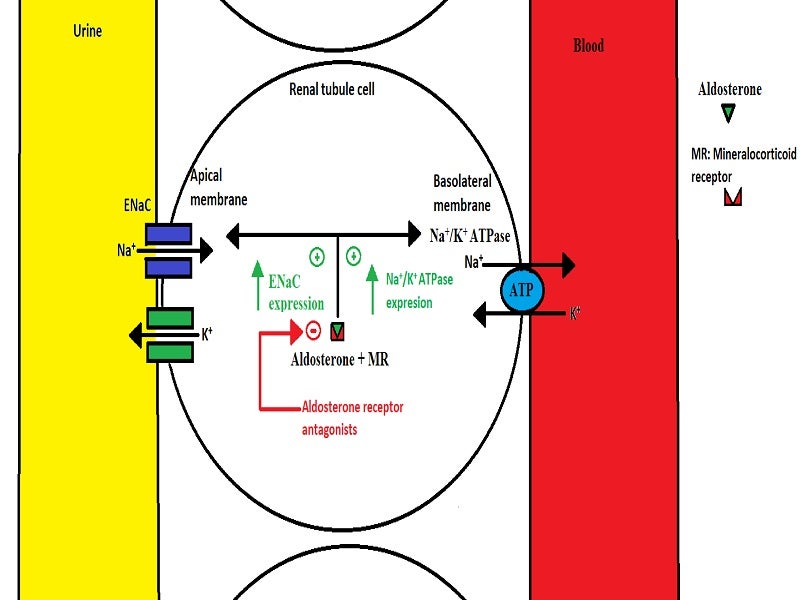
Finerenone is a selective antagonist of the mineralocorticoid receptor (MR). Activated by aldosterone and cortisol, the nonsteroidal MRA regulates gene transcription. The overexpression of the MR is believed to contribute to fibrosis and inflammation.
Finerenone selectively binds to the MR receptor, inhibiting MR-mediated sodium reabsorption as well as MR overexpression in both epithelial (kidney) and nonepithelial (heart and blood vessel) tissues.
Finerenone has a high potency and selectivity for the MR, but no significant affinity for androgen, progesterone, oestrogen or glucocorticoid receptors.
Clinical trials on KERENDIA
KERENDIA’s approval was supported by the positive outcomes of a double-blind, multi-centre, randomised, placebo-controlled, pivotal Phase III clinical trial, FIDELIO-DKD, in patients with CKD associated with T2D.
The study was part of a comprehensive finerenone clinical trial programme that enrolled 13,000 patients with a wide spectrum of disease severity, including those with early kidney impairment and those in more advanced stages of kidney disease.
In the FIDELIO-DKD trial, 5,674 patients were randomly assigned to receive either KERENDIA (N=2,833) or placebo (N=2,841). The starting dose was determined by screening the eGFR, which could be titrated, with a daily target dose of 20mg.
The study’s primary goal was to determine KERENDIA’s efficacy in reducing the incidence of a sustained decline in kidney function, progression to kidney failure or renal death compared to a placebo.
Results showed that 17.8% of the KERENDIA-treated patients demonstrated the incidence of a sustained decline in kidney function, progression to kidney failure or renal death compared to 21.1% of patients in the placebo group.
KERENDIA also lowered the incidence of cardiovascular death, non-fatal MI, non-fatal stroke or hospitalisation for heart failure.
The adverse reactions reported in patients treated with KERENDIA during the trial include hyperkalaemia, hypotension and hyponatraemia.
Another randomised, double-blind, placebo-controlled, parallel-group, event-driven Phase III clinical trial named FIGARO-DKD was conducted under the finerenone study programme to determine the cardiovascular outcomes in patients with CKD and T2D.
7,400 patients from more than 1,000 locations across 47 countries, including Canada, were enrolled in the study.
The study showed that finerenone significantly reduced the risk of cardiovascular death and non-fatal cardiovascular events in patients compared to placebo together with the standard of care.
Bayer subsequently initiated a multi-centre, randomised, double-blind, placebo-controlled Phase III study, FINE ARTS-HF, to determine finerenone’s superiority over placebo in more than 5,500 patients with symptomatic heart failure and a left ventricular ejection fraction of 40% or more.
The trial’s objective was to demonstrate finerenone’s superiority over placebo in reducing the rate of cardiovascular death and total heart failure events.