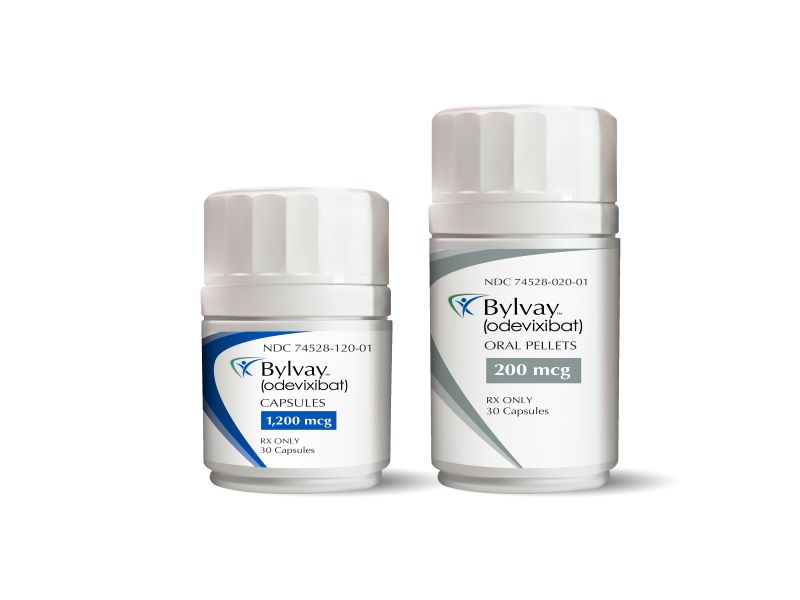
Bylvay™ (odevixibat) is the first drug indicated for the treatment of pruritus in patients aged three months and older with progressive familial intrahepatic cholestasis (PFIC) and cholestatic pruritus in patients aged 12 months and older with Alagille syndrome (ALGS).
Developed by US-based biopharmaceutical company Albireo Pharma, Bylvay moved to the rare disease portfolio of Ipsen, a France-based biopharmaceutical company, following the acquisition of Albireo by the latter in March 2023. Besides Bylvay, two more clinical-stage assets in Albireo’s pipeline A3907 and A2342 also moved to Ipsen’s portfolio as part of the transaction.
Bylvay is available as 200mcg and 600mcg oral pellets, as well as capsules of 400mcg and 1200mcg dosage strengths.
Regulatory approvals for Bylvay
Albireo Pharma submitted a new drug application (NDA) to the US Food and Drug Administration (FDA) and a marketing authorisation application (MAA) to the European Medicines Agency (EMA) seeking approval of odevixibat for the treatment of patients with PFIC, in December 2020.
The FDA accepted the NDA for priority review in January 2021 while the EMA’s Committee for Medicinal Products for Human Use (CHMP) recommended the drug for approval in May 2021.
Odevixibat secured approval for the condition from the FDA and the EMA in July 2021.
The drug’s commercial launch in the European Union (EU) began with Germany, where the drug was made available in September 2021. The drug was then launched in Italy, France, and Belgium. Furthermore, the drug was approved by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) for the treatment of all types of PFIC in September 2021.
Albireo filed supplemental regulatory applications for Bylvay to treat ALGS in the EU and the US in December 2022. The FDA accepted it for priority review in February 2023 and approved it for the condition in June 2023.
The EMA’s CHMP adopted a positive opinion recommending the approval of Bylvay to treat cholestatic pruritus in patients with ALGS aged six months or older in July 2023.
Bylvay was granted orphan exclusivity for the treatment of PFIC and holds orphan drug designations for ALGS and biliary atresia (BA) in the US and the EU. Biliary atresia is a rare paediatric cholestatic disease that can lead to cirrhosis and liver failure.
Bylvay is currently in late-stage development with the Phase III clinical trial BOLD for BA.
PFIC and ALGS causes and symptoms
PFIC is a rare, progressive, life-threatening liver disorder affecting young children. It is a hereditary disease in which the liver cells can’t produce and secrete bile properly, resulting in the build-up of bile in the liver cells called cholestasis.
Cholestasis can damage the liver, causing cirrhosis and liver failure within the first ten years of life. Three known types of PFIC exist, namely PFIC type 1, type 2 and type 3.
The most prominent feature of the disorder is severe and debilitating pruritus or intense itching. Other symptoms include jaundice, poor weight gain, and slowed growth.
PFIC affects one to two children for every 100,000 live births. Both genders are equally affected by the disease.
ALGS is a rare genetic disorder affecting multiple organ systems, including the liver, heart, skeleton, eyes, and kidneys.
Liver damage can result from narrowed or malformed bile ducts, leading to toxic bile acid build-up and progressive liver disease. Approximately 95% of patients experience chronic cholestasis within the first three months of life while 88% also experience severe pruritus.
The global incidence of ALGS is three in 100,000 live births, with 1,300 patients eligible for ileal bile acid transport inhibitor (IBATi) treatment in the US.
The majority of Alagille syndrome patients have mutations in one copy of the JAG1 gene, whereas several have NOTCH2 gene mutations, which can be inherited in an autosomal dominant pattern.
Bile flow obstruction, jaundice, poor weight gain, and intense itching are the common symptoms of ALGS. Heart murmurs, congenital heart abnormalities, vertebral variations, anterior embryotoxon thickness, and unusual facial traits are among the other signs.
Bylvay mechanism of action
Bylvay is a once-daily, non-systemic, reversible inhibitor of ileal bile acid transporter (IBAT), which decreases the reuptake of bile salts from the terminal ileum into the hepatic portal circulation. The therapy acts locally in the small intestine.
The elimination of bile acids from the enterohepatic circulation reduces bile acid levels in serum and the liver.
Clinical trials on Bylvay
The FDA’s approval of Bylvay for pruritus associated with PFIC was supported by the PEDFIC I and PEDFIC II clinical trials, which were the largest, global, Phase III clinical trials ever performed in the PFIC space.
PEDFIC I was a 24-week, randomised, double-blind, placebo-controlled trial that enrolled 62 paediatric patients aged between six months and 17 years with PFIC type 1 or type 2 and severe itching.
The patients were randomised to receive either a placebo or 40mcg/kg or 120mcg/kg of odevixibat.
The study’s primary endpoints were the difference in a patient’s scratching as observed by their caregiver twice a day and assessed on a five-point ordinal scale, as well as serum bile acid responses.
The results of the study showed that patients treated with odevixibat achieved a significant decline in itching or scratching and reduced serum bile acid responses. Approximately 53.5% of patients in the odevixibat arms showed a significant reduction in pruritus, compared to 28.7% in the placebo arm.
Odevixibat’s safety was assessed in PEDFIC II, a 72-week, long-term, open-label, single-arm extension study that enrolled 79 participants with PFIC types 1, 2 or 3 and aged between four months and 25 years. The patients received 120mcg/kg of the drug once a day.
PEDFIC II confirmed that Bylvay showed sustained reductions in serum bile acids and improvements in pruritus, growth and other liver function markers in patients treated for up to 48 weeks.
The FDA’s approval of Bylvay for ALGS condition was supported by a Phase Ⅲ double-blind, randomised, placebo-controlled clinical trial named ASSERT.
ASSERT was designed to evaluate the safety and efficacy of 120µg/kg/day Bylvay for 24 weeks in reducing pruritus in ALGS patients across 32 sites in North America, Europe, the Middle East, and the Asia Pacific.
The study met the primary endpoint demonstrating a substantial reduction in pruritus as measured by the PRUCISION Observer-Reported Outcome scratching score on a scale of zero to four points from baseline compared to placebo at month six. The trial also achieved the important secondary endpoint of lowering serum bile acid content.