Livmarli™ (maralixibat) is indicated for the treatment of cholestatic pruritus associated with Alagille syndrome (ALGS) in patients aged one and older.
Mirum Pharmaceuticals, a biopharmaceutical company based in the US, developed the drug following the acquisition of its global development and commercialisation rights from Shire Pharmaceuticals in November 2018.
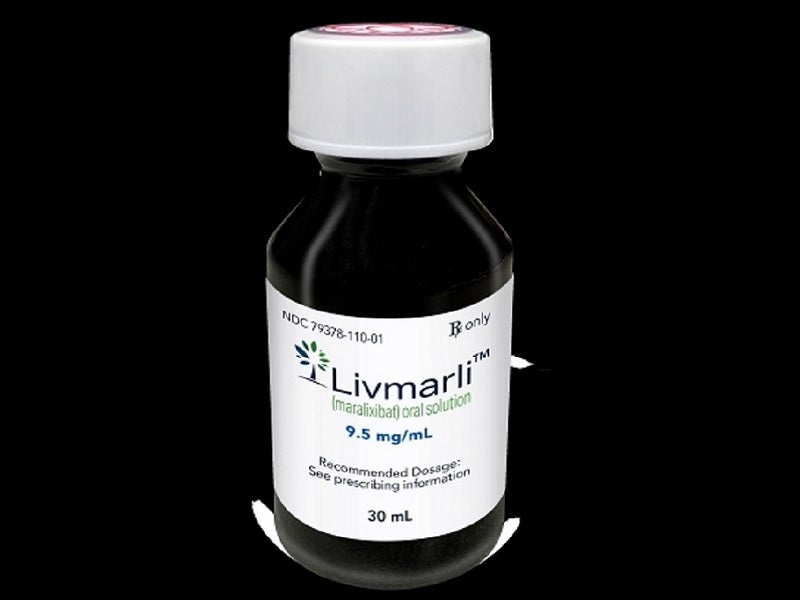
Livmarli™ is available as a clear, colourless to yellow solution in 9.5mg of maralixibat per ml dosage strength for oral administration.
Regulatory approvals for Livmarli
The US Food and Drug Administration (FDA) granted breakthrough therapy designation (BTD) to maralixibat for the treatment of pruritus associated with ALGS in October 2019.
Mirum initiated a rolling submission of a new drug application (NDA) for maralixibat to the FDA and launched an expanded access programme (EAP) for the drug for the condition in September 2020.
The submission of a rolling NDA for maralixibat was completed in February 2021 and was accepted for priority review in March 2021. The FDA granted approval to Livmarli™ (maralixibat) oral solution in September 2021. The company submitted a marketing authorisation application (MAA) for maralixibat to the European Medicines Agency (EMA) for the treatment of cholestatic liver disease in patients with ALGS in the same month of 2021.
Maralixibat is also being reviewed for the treatment of progressive familial intrahepatic cholestasis type 2 (PFIC2), also known as bile salt export pump (BSEP) deficiency, by the EMA.
The drug also holds the FDA’s rare paediatric disease designation for ALGS and orphan drug designation for the treatment of patients with PFIC and ALGS.
ALGS causes and symptoms
ALGS is a rare genetic disorder that causes abnormalities in bile ducts, leading to an accumulation of bile in the liver and consequent progressive liver disease. The condition can affect the liver, heart, skeleton, and eyes.
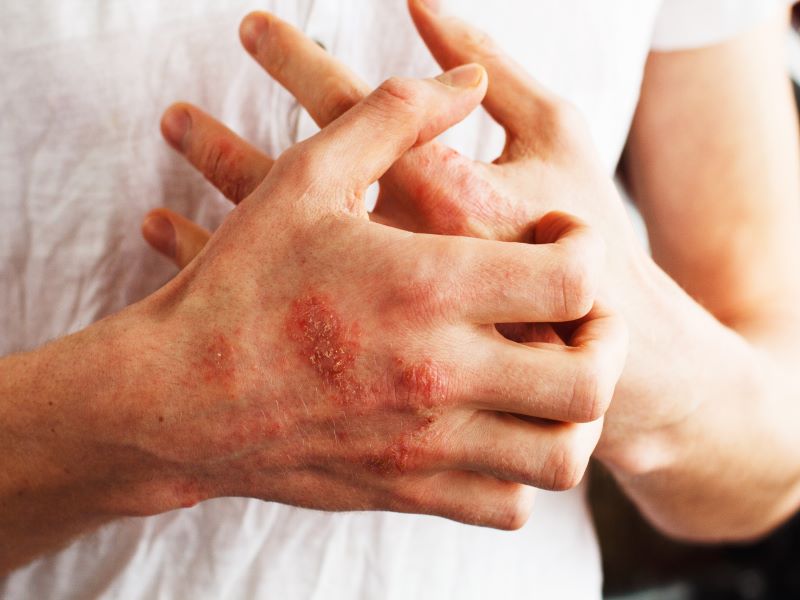
Pruritus is a common symptom in patients with ALGS. The pathophysiology of pruritus in patients with ALGS is, however, not completely understood. Cholestasis in ALGS is associated with pruritus which is one of the most common indications for liver transplant in ALGS.
Cholestatic pruritus is a serious, unremitting and debilitating condition that affects children with ALGS, disrupting their sleep. The children suffer from bleeding and skin damage due to constant scratching.
Livmarli’s mechanism of action
Livmarli™ (maralixibat) oral solution is a new, once-daily, minimally absorbed, reversible inhibitor of ileal bile acid transporter (IBAT).
It inhibits the apical sodium-dependent bile acid transporter (ASBT), causing excess excretion of bile acids into the faeces due to a reduction in bile acid resorption from the terminal ileum. The condition leads to a systemic decline in bile acid levels in the liver and reduces bile acid-mediated liver damage and associated complications.
Clinical trials on Livmarli
The FDA’s approval of Livmarli was based on the positive outcomes of a pivotal clinical study named ICONIC, as well as the data from supportive studies conducted for five years, on 86 recruited patients with ALGS.
The long-term, randomised, controlled phase two B ICONIC clinical study involved an 18-week open-label treatment period, a four-week randomised, double-blind, placebo-controlled drug withdrawal period, followed by a 26-week open-label treatment period and a long-term open-label extension period.
The ICONIC study enrolled 31 patients who were initially treated with maralixibat for 18 weeks before being randomised to receive either placebo (n=16) or maralixibat (n=13) for four weeks. The patients were treated with maralixibat for the remaining 48 weeks, upon completion of the randomised drug-withdrawal period.
Out of the 31 recruited patients, 29 completed the drug withdrawal period, while 28 completed the following 48-week treatment. Primary results showed that maralixibat demonstrated significant reductions in serum bile acids, pruritus, and xanthomas at week 48. The drug was generally well-tolerated by patients.
Treatment-emergent adverse events (TEAEs) were reported in 30 patients, among who four reported serious TEAEs that were unrelated to maralixibat and two reported TEAEs leading to discontinuation, also unrelated to the therapy. Diarrhoea and abdominal pain were the most frequent TEAEs reported in the patients during the clinical trial.