
Cell therapies, specifically chimeric-antigen receptor T (CAR-T) cells, have had a tremendously positive impact on the treatment of many blood malignancies, including B-cell non-Hodgkin’s lymphoma (NHL), acute lymphocytic leukaemia and multiple myeloma. All such products are being used in the relapsed or refractory (R/R) setting, but physicians anticipate their incorporation into the frontline treatment. T-cell lymphomas (TCL) are a diverse type of NHL for which no cell therapies have been approved, with many currently under clinical trials.
GlobalData’s Pharma Intelligence Centre lists 51 ongoing cell therapy clinical trials in TCLs. As was the case in the early stages of B-NHL CAR-T trials, most of the current clinical trials for T-NHL are academic. Of interest is the fact that China is highly active in such trials, with around half of institution-led trials being conducted in China. In the US, the UNC Lineberger Comprehensive Cancer Centre and the Baylor College of Medicine have initiated the most cell therapy TCL trials.
Of the company-sponsored trials, no trial is in a strictly pivotal or registrational stage, but GlobalData expects that certain products in Phase I/II are well-positioned for a swift transition to a pivotal trial upon encouraging early-phase results. Such products include the anti-TRBC1 CAR-T cells, Autolus Therapeutics’ AUTO4, and the cytotoxic T-lymphocyte (CTL) products, Boryung ViGenCell‘s VT-EBV-N and Eutilex’s EBViNT, among others (Figure 1). Of these, AUTO4 has secured the special designation of the UK’s Innovative Licensing and Access Pathway.
Targeting T-cell-driven blood cancers presents many challenges, the chief among them being the deleterious effect of normal T-cell depletion, unlike B-cells, whose depletion has proven a lot more tolerable in the clinic. As such, classic T-cell antigens such as CD4, CD7 and CD30, targeted by many of the early-phase cell therapy products in the pipeline, have raised significant key opinion leader (KOL) concerns regarding anticipated toxicity. KOLs interviewed by GlobalData have, therefore, praised preferential targeting approaches, such as targeting TRBC1 with AUTO4, which aims to spare a subset of normal T-cells in an effort to minimise toxicity.
A high attrition rate in late-stage TCL cell therapy trials is to be expected due to the challenges described above. In addition, the relative rarity of the disease could not allow space for numerous products to compete, as has been the case with B-NHL. That is not to say that commercial success is unlikely for a product with the first-mover advantage. According to GlobalData estimates, a successful product that could be used for both cutaneous and peripheral TCL patients could achieve peak sales in the range of $100m to 200m.
What the future holds for cell therapies in TCL is still largely uncertain. B-cell CAR-T approaches, which are now all autologous, are expected to be replaced by allogeneic products in the future. If this happens, current allogeneic pipeline products for TCLs, despite being very few in number, are expected to have a competitive advantage in the later stages of their lifecycle.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData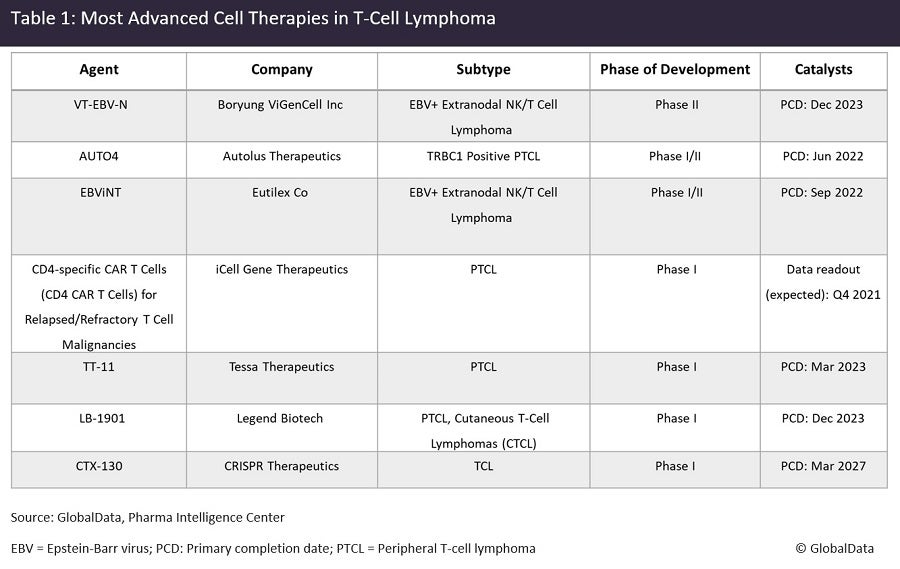
Cell & Gene Therapy Coverage on Clinical Trials Arena supported by Cytiva.
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.





