This week focuses on cervical cancer prevention in the UK. Since last year’s “Cervical Cancer Trials Peak for 2021” analyst briefing, GlobalData has recorded a decrease in the number of cervical cancer trials for 2022. Cervical cancer is the fourth most common cancer in women, with a five-year survival rate of 66%. Cases are largely caused by human papillomavirus (HPV) infection. Cervical screenings help prevent the development of cervical cancer by assessing cervical cells for abnormalities and testing for HPV.
In November 2021, The Lancet published a study showing how the national HPV vaccination program in England was highly successful at decreasing the risk of developing cervical cancer. The vaccination program focused on vaccinating girls ages 12–13 years, and results showed a substantial reduction in cervical cancer cases and the incidence of cervical intraepithelial neoplasia III (CIN3) in young women. Global immunisation with the HPV vaccine has been low, with a recent PubMed study showing that the immunisation coverage is approximately 12.2%. According to recent official figures, the UK is currently facing a record ten-year high of women not being screened for cervical cancer.
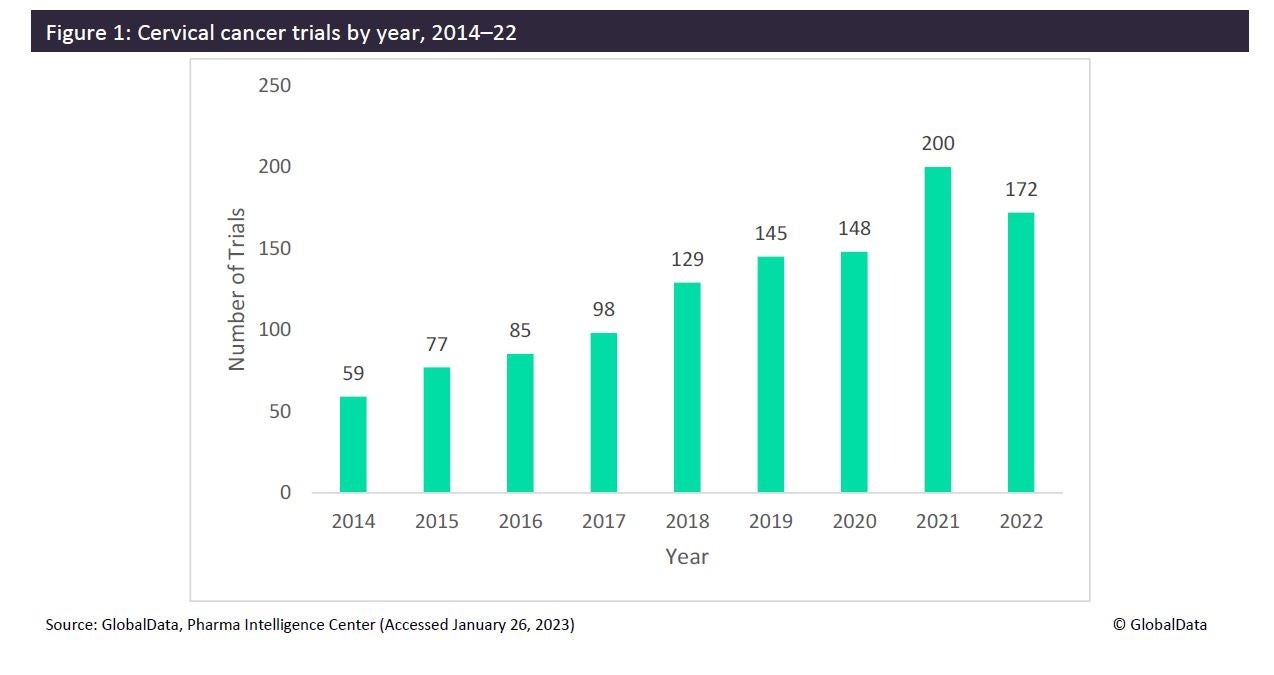
According to GlobalData’s Clinical Trials Database, the number of cervical cancer trials peaked at 200 trials in 2021 (Figure 1) and then decreased to 172 trials in 2022. Many of these trials focused on the efficacy of vaccination programs within specific countries while others focused on treatment therapies. Globally, the US had the greatest portion of cervical cancer trials with 31.8%, followed by China with 28.0%. Top sponsors for 2022 included Peking Union Medical College with five trials, as well as the University of Texas MD Anderson Cancer Center, Akeso, and the National Cancer Institute US, with four trials each.