As of 31 January, the Clinical Trials Information System (CTIS) became the single portal that sponsors and regulators must navigate to submit new clinical trial applications in the EU. This landmark shake-up retires Europe’s previous clinical trial database, EudraCT, after almost two decades. EudraCT encompassed a multi-step regulatory approval process comprising separate applications to National Competent Authorities (NCAs), as well as ethic committees in each EU country. Now, with CTIS, a single document and application can achieve authorization in up to 30 EU/European Economic Area (EEA) countries.
The aspiration is to streamline regulatory approval of clinical trials in the EU. Centralising all clinical trial submission data within CTIS is intended to alleviate the administrative burden of the regulatory process, improving its efficiency and consistency. Trial transparency will also improve as all data regarding clinical trial authorisation through CTIS will be publicly available (unless confidentiality is justified).
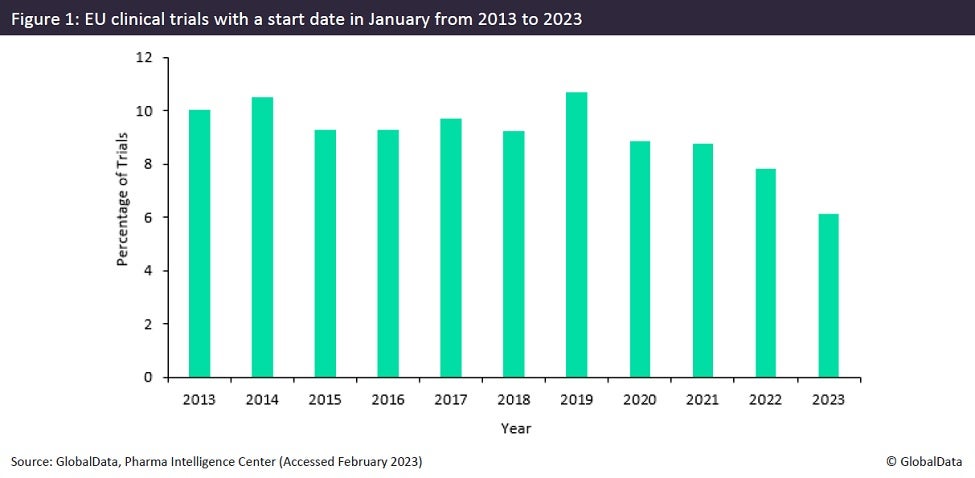
Despite the promise of simpler and faster clinical trial authorisation with CTIS, data obtained from GlobalData’s Trials Intelligence platform indicates that clinical trials initiated in the EU last month hit a record low on the heels of compulsory CTIS implementation (Figure 1). This trend is not replicated in the other main clinical trial markets of North America or Asia-Pacific. This alludes to a correlation between sponsors’ trepidations regarding CTIS, and Europe’s ten-year low January trial initiation.
Feedback received last year following the launch of CTIS included a host of software issues relating to the usability and functionality of the system. Concerns ranged from reported blocking issues to poor adaptability for specific study designs such as platform trials. The EMA has since assured users that 80% of blocking issues have been resolved; however, the initial learning curve will inevitably lead to a temporary increased workload, which can be daunting. Ultimately, time will tell if the CTIS portal was the right choice over popular suggestions among users, such as a transmission from system-to-system approach.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData




