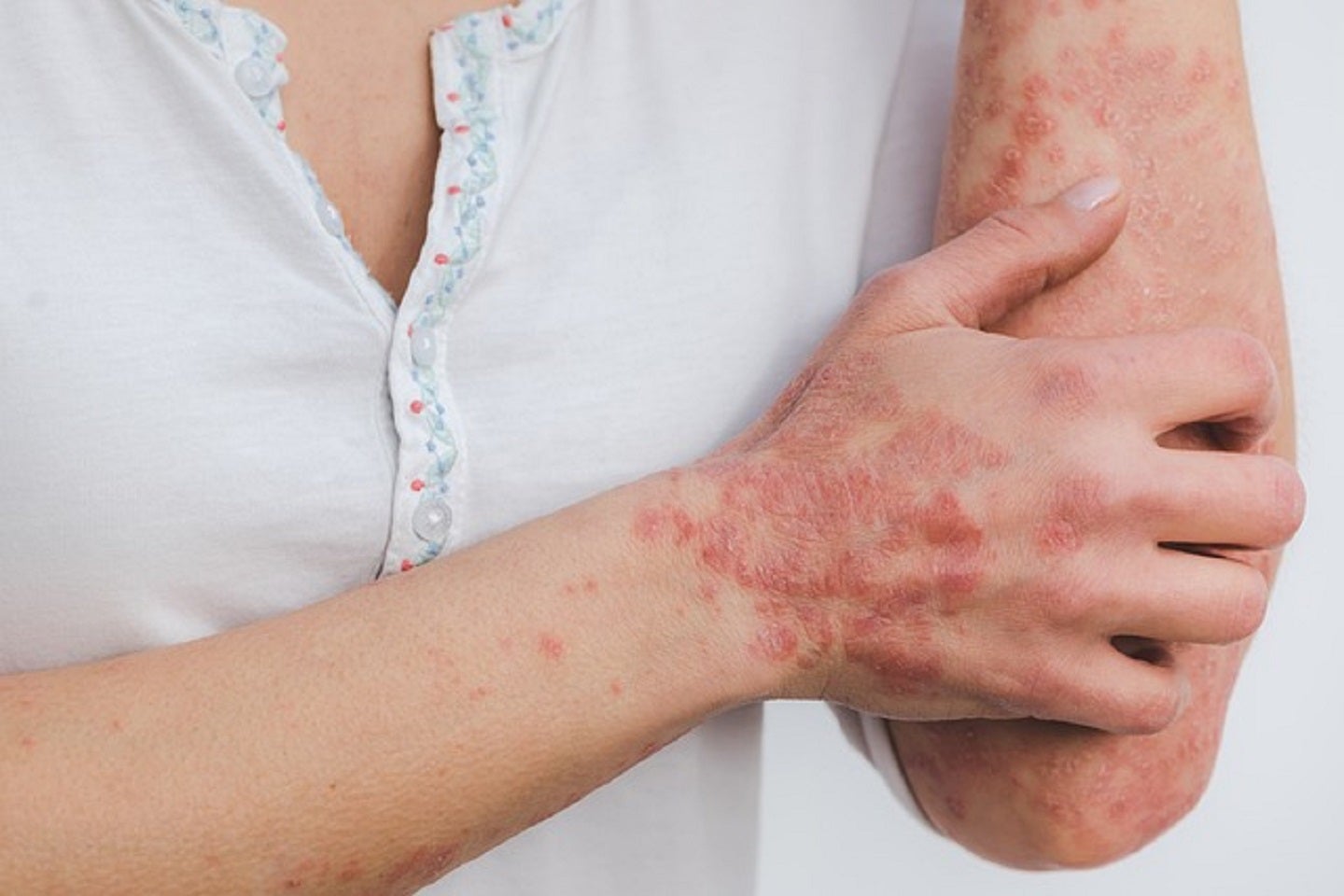
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has granted positive opinion for Can-Fite BioPharma’s registration plan submission for Phase III trial of Piclidenoson for the treatment of patients with moderate to severe psoriasis.
The agency has accepted the pivotal trial and the safety of the 3mg dose of Piclidenoson given two times a day.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Can-Fite plans to commence the prospective, double-blind, placebo-controlled and randomised trial of Piclidenoson to demonstrate its clinical efficacy and safety.
This will help the company submit a marketing authorization application.
Can-Fite CEO Dr Pnina Fishman said: “Piclidenoson’s oral dosage and excellent safety record combined with its progressive effectiveness over time make it ideally suited for the treatment of psoriasis, a chronic disease.
“Should this market registration study produce positive results similar to our COMFORT study, we believe Piclidenoson will be well positioned in a very large market which needs more safe and effective oral drug options.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company’s COMFORT study of Piclidenoson was found to meet its primary endpoint with statistically significant improvement against placebo in patients with psoriasis.
The Phase III multicentre, randomised, placebo- and active-controlled, double-blind study evaluated the efficacy and safety of the therapy in over 400 adult patients with moderate to severe plaque psoriasis.
It also showed excellent safety profile in psoriasis patients.
The company has also submitted the chemistry, manufacturing, and controls (CMC), nonclinical data, and clinical pharmacology data to the agency.
In addition, it is submitting a comparable data package to the US Food and Drug Administration (FDA).





