American biopharmaceutical giant Bristol Myers Squibb (BMS) announced on 28 March 2023, the European Commission (EC) approval of Sotyktu (deucravacitinib), its first-in-class, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor for adults with moderate-to-severe plaque psoriasis (PsO) who are eligible to receive systemic therapy or phototherapy.
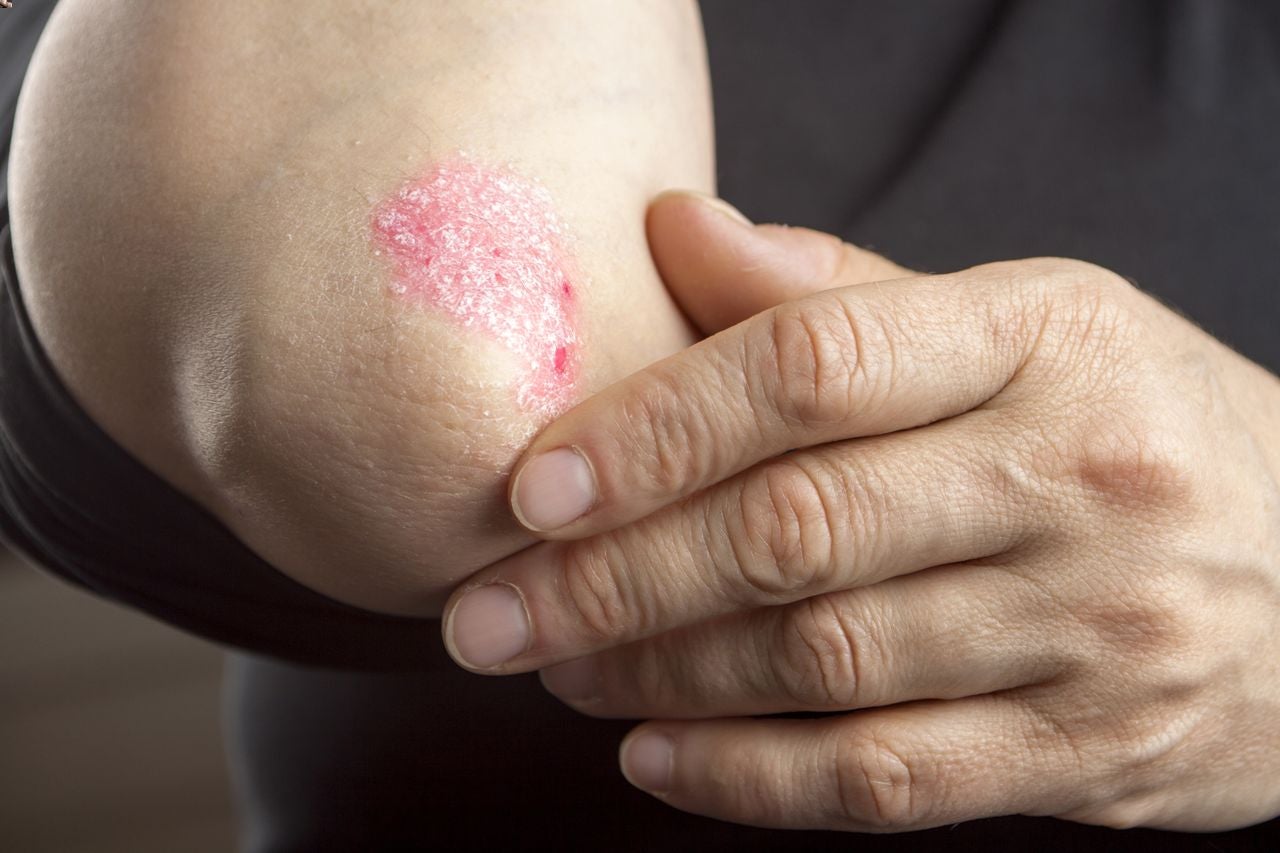
PsO is a chronic and systemic inflammatory dermatological condition, which affects 2%–3% of the world’s population and is linked to physical and mental effects and debilitated quality of life, with Europe having the highest prevalence globally. BMS’s Sotyktu represents a new class of small molecules with a unique mechanism of action and is hailed as a landmark achievement due to the prior absence of a proven once-daily, symptom-relieving, and tolerable therapeutic alternative in the region.
Following intake and through a high degree of sensitivity, Sotyktu selectively targets and binds the pseudokinase domain of TYK2, thus allosterically inhibiting signalling of interleukin 23 (IL-23), IL-12, and Type 1 interferons (IFN), key inflammatory cytokines in the pathogenesis of psoriasis.
The EC has based its approval of the drug on results from BMS’s global, randomized, double-blind Phase III POETYK PSO-1 (NCT03624127) and POETYK PSO-2 (NCT03611751) clinical trials which exhibited superior efficacy of Sotyktu compared to a placebo and Amgen’s oral Otezla (apremilast), its direct competitor, at weeks 16 and 24, with responses maintained over a period of 52 weeks. The results of both clinical trials were evaluated in relation to the Psoriasis Area and Severity Index (PASI) and the static Physician’s Global Assessment (sPGA), with a PASI 75 and a sPGA score of 0 (clear) or 1 (almost clear) at week 16 versus the placebo and Otezla arms, being the co-primary endpoints. The clinical trials established that a significantly higher number of Sotyktu-treated patients with moderate-to-severe PsO had achieved an sPGA score of 0/1 and PASI 75 and 90 response, with the positive clinical outcomes being maintained over 52 weeks. BMS reported that in the POETYK PSO-2 trial, 80% of the adult patients who continued the Sotyktu course maintained PASI 75 response compared to 31% of those who were withdrawn from the drug. Further data points from the POETYK PSO long-term extension trial (LTE) (NCT04036435), in which patients could enrol after the 52-week PSO-1 and 2 trials, reinforced the European approval after a consistent safety profile in patients following a continuous treatment duration of three years with the open-label Sotyktu 6mg once-daily PsO drug.
According to GlobalData’s Drugs Database Pharma Intelligence Centre, with an analyst consensus global sales forecast of $2.9bn by 2029, Sotyktu is set to become a blockbuster for the treatment of PsO, directly competing against formidable and established biologics in the space, including AbbVie’s highly acclaimed Skyrizi (risankizumab). However, BMS has reported that, at therapeutic doses, Sotyktu’s TYK2 mechanism of action does not inhibit Janus kinase (JAK) 1, 2, or 3 and it has not yet been established whether it may be associated with the observed or potential adverse reactions of JAK inhibition. Nonetheless, the inhibition of TYK2 can potentially affect a smaller number of inflammatory cytokine pathways when compared to other JAK inhibitors. Thus, it is expected that the selective inhibition of TYK2 will prevent the toxicities that are commonly linked with JAK inhibitors, rendering Sotyktu a safe option (Yang, Oak, and Elewski, 2021).
Overall, GlobalData understands that the positive clinical trial results and its new European arena underpin Sotyktu’s outlook as a leading drug in the crowded and growing PsO space.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData