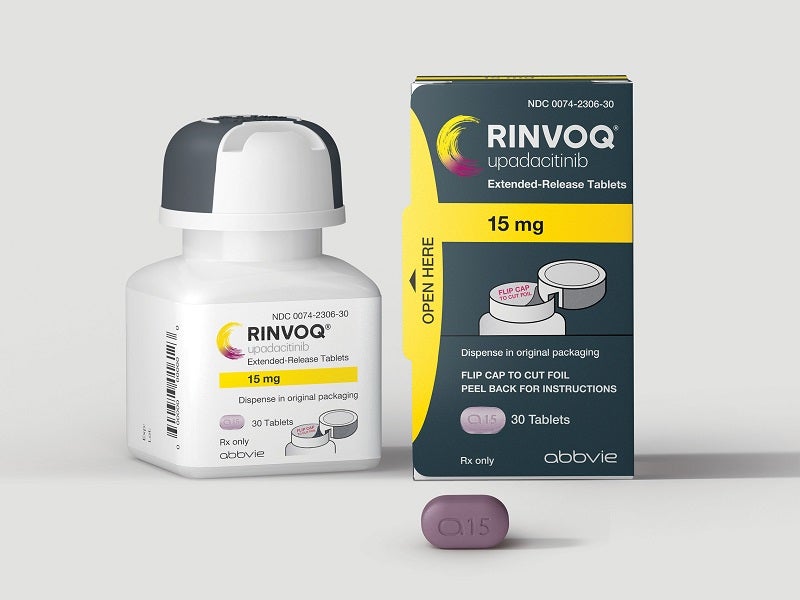
Rinvoq (upadacitinib) is an oral, once-daily Janus Kinase (JAK) inhibitor indicated to treat moderately to severely active Crohn’s disease, moderate to severe rheumatoid arthritis (RA), active psoriatic arthritis (PsA), active ankylosing spondylitis (AS), active non-radiographic axial spondyloarthritis (nr-axSpA), and moderate to severe ulcerative colitis (UC) in adults who have undergone an inadequate response to one or more tumour necrosis factor (TNF) blockers.
The drug is also indicated to treat moderate to severe atopic dermatitis, which is not well controlled with other pills or injections, including biologics.
Developed by US-based pharmaceutical company Abbvie, Rinvoq is available in 15mg, 30mg and 45mg dosage strengths as biconvex oblong-shaped extended-release tablets. The 15mg tablet is purple, 30mg is red, and 45mg is yellow to mottled yellow in colour.
Regulatory approvals for Rinvoq
Rinvoq received its initial US Food and Drug Administration (FDA) approval for the treatment of RA in August 2019.
In December 2021, the FDA approved the drug for PsA. Rinvoq further received approvals for atopic dermatitis, UC, AS and nr-axSpA in 2022.
The European Commission (EC) approved Rinvoq to treat moderately to severely active Crohn’s disease in April 2023, making it the first JAK inhibitor approved for the condition in Europe. Rinvoq received approval for the same condition in the US in May 2023.
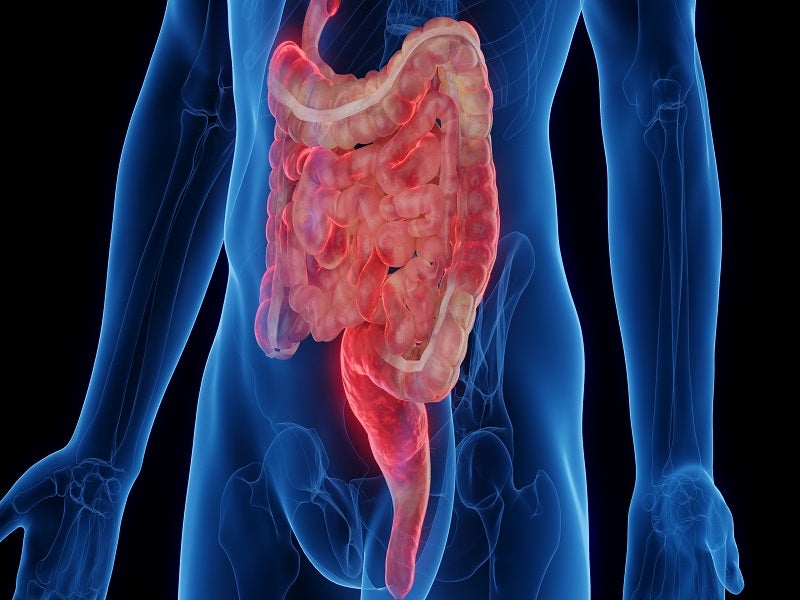
Crohn’s disease causes and symptoms
Crohn’s disease is a type of inflammatory bowel disease (IBD), which causes inflammation in the tissues in the digestive tract, specifically small and large intestines.
The disease may lead to abdominal pain, severe diarrhoea, fatigue, weight loss and malnutrition. The condition can often be disruptive and debilitating and, in some cases, may lead to severe complications that require urgent medical care, including surgery.
Crohn’s disease is generally seen in younger people. It can, however, also appear in individuals of any age, including children. Cigarette smokers are more prone to the disease than non-smokers.
Severe patients can show symptoms outside the intestinal tract, including inflammation of skin, eyes, and joints, inflammation of liver or bile ducts, kidney stones, anaemia, and delayed growth.
Rinvoq’s mechanism of action
Rinnvoq is a selective JAK inhibitor that works inside cells to block certain signals that are believed to cause inflammation. It is a small molecule that targets JAK and inhibits the signalling of the JAK-signal transducer and activator of the transcription (STAT) pathway, a signalling network utilised by proinflammatory cytokines.
By binding to JAK, Rinvoq is believed to block the phosphorylation and activation of STATs, disrupting the proinflammatory cytokine signalling cascade.
Clinical trials on Rinvoq
The approval of Rinvoq for moderately to severely active Crohn’s disease was based on two induction clinical studies, namely U-EXCEED and U-EXCEL, and one maintenance study, U-ENDURE.
The three Phase III multi-centre, randomised, double-blind, placebo-controlled clinical trials evaluated the efficacy and safety of Rinvoq (upadacitinib) 45mg as induction therapy and Rinvoq 15mg and 30mg as maintenance therapy in patients with moderately to severely active Crohn’s disease.
The co-primary endpoints of the studies were endoscopic response (visible reduction of intestinal lining damage) and clinical remission.
The studies showed a significant statistical improvement with Rinvoq (upadacitinib) 45mg in the induction studies, and Rinvoq 15mg and 30mg in the maintenance study, compared to placebo.
In the two induction studies, 34% and 46% of patients treated with Rinvoq 45mg achieved an endoscopic response compared to 3% and 13% of patients respectively receiving a placebo at week 12, respectively.
In the maintenance study, 28% and 41% of patients who received Rinvoq 15mg and 30mg achieved endoscopic response at week 52, respectively, versus 7% of patients treated with a placebo.
Clinical remission was achieved in 36% and 46% of patients who received Rinvoq in the two induction studies, at 12 weeks, compared to 18% and 23% of patients who received a placebo.
Furthermore, in the maintenance study, 42% and 55% of individuals treated with Rinvoq 15mg and 30mg achieved clinical remission at 52 weeks, respectively, versus 14% of patients treated with a placebo.
Overall, the safety profile observed in patients with Crohn’s disease receiving Rinvoq was consistent with the known safety profile for Rinvoq in other indications.