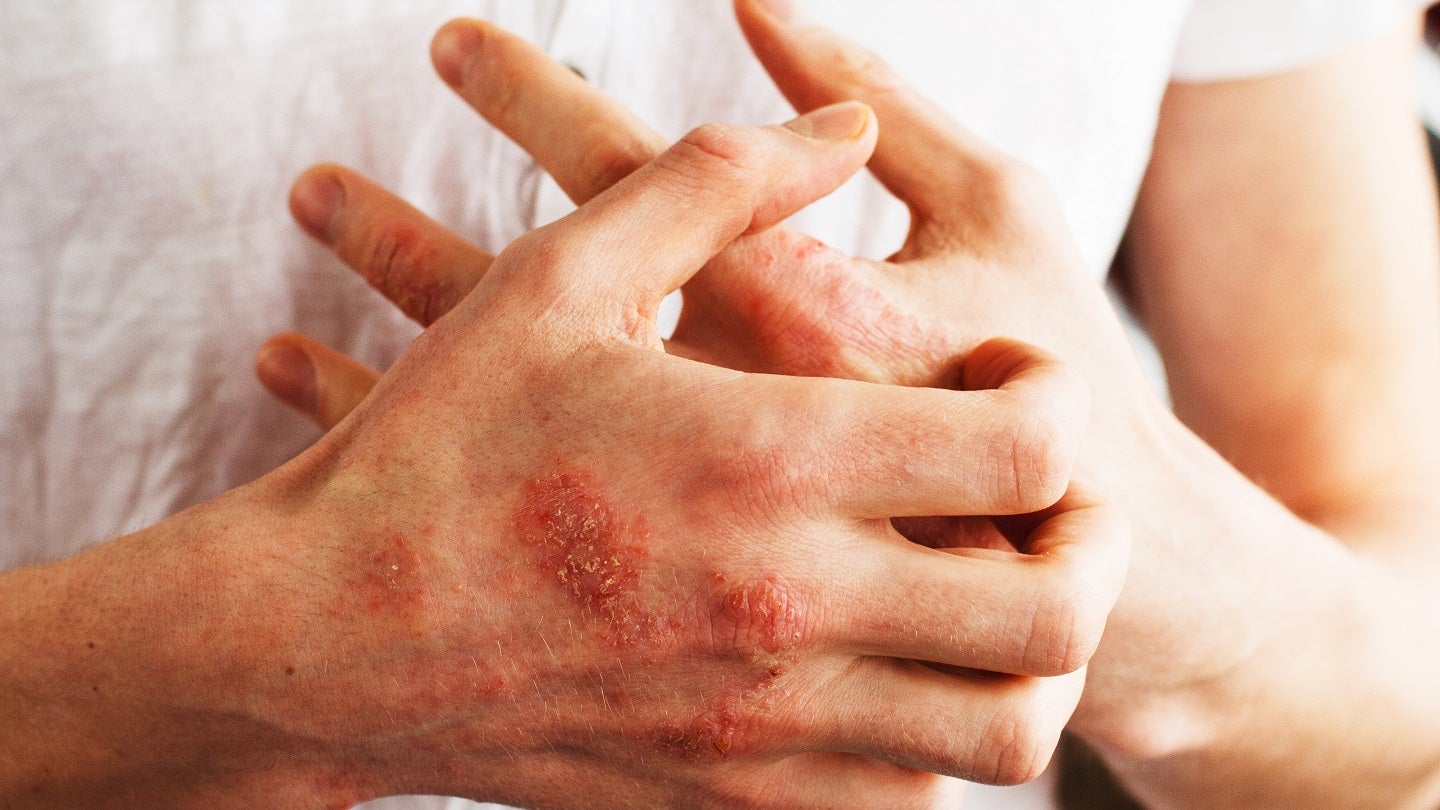
Johnson & Johnson subsidiary Janssen Pharmaceutical Companies (Janssen) has reported that its Phase IIb FRONTIER 1 clinical trial of JNJ-2113 for plaque psoriasis (PsO) met both its primary and secondary efficacy endpoints after 16 weeks.
The study is designed to evaluate the use of the oral interleukin-23 receptor (IL-23R) antagonist peptide JNJ-2113 to treat adults with moderate-to-severe plaque PsO.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Its primary endpoint is the percentage improvement in skin lesions as measured by the Psoriasis Area and Severity Index (PASI) with a score of 75 compared with placebo at week 16.
Secondary efficacy endpoints included achieving PASI 90 and PASI 100 against placebo.
Complete improvement in skin clearance was observed at the highest dose level tested against a placebo at week 16.
In addition, most patients who received treatment with JNJ-2113 achieved PASI 75, as well as PASI 90 and PASI 100.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataJNJ-2113 was well-tolerated, although one or more adverse events were reported.
Janssen vice-president and immunodermatology disease area stronghold leader Lloyd Miller said: “The development of a novel oral therapy that specifically targets IL-23R could potentially change the treatment paradigm for patients living with moderate-to-severe plaque psoriasis.
“Until now, advanced psoriasis treatments have been largely limited to injectable biologics.
“An oral therapy that can uniquely inhibit the IL-23 pathway by directly targeting the IL-23 receptor could help address the needs and preferences of patients, and may offer greater freedom, with the aim of driving greater adoption of advanced treatment.”
The results from this trial will enable Janssen to advance its Phase III clinical development of JNJ-2113 in treating moderate-to-severe plaque PsO, as well as begin a Phase IIb study to treat adults with ulcerative colitis.
Last month, the company presented positive data from its ‘proof-of-concept’ Phase II trial of nipocalimab in pregnant individuals at high risk of developing haemolytic disease of the foetus and newborn.





