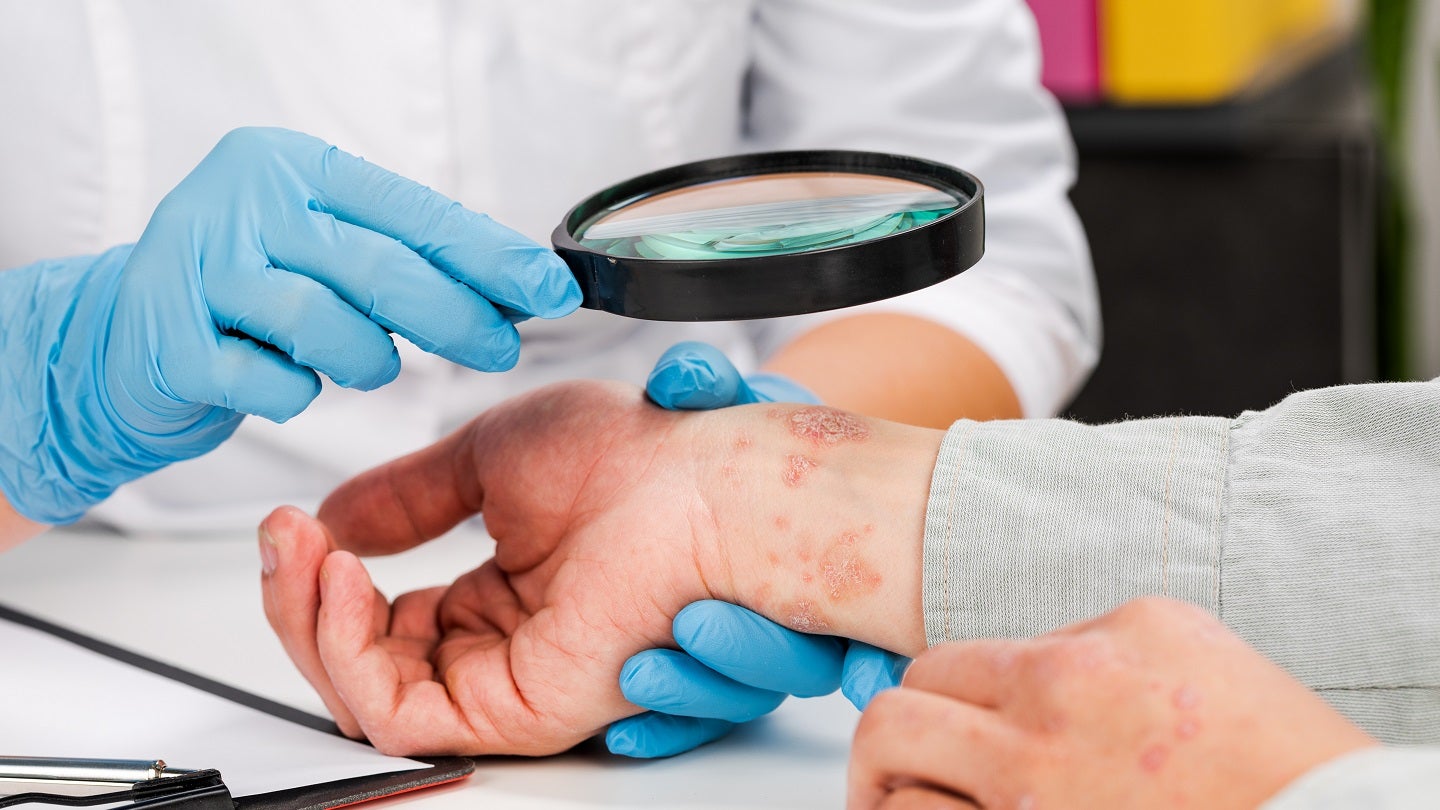
InnoCare Pharma has dosed the first patient in a clinical trial of ICP-488, a tyrosine kinase 2 (TYK2) JH2 allosteric inhibitor used for the treatment of psoriasis, in China.
The ongoing study intends to assess the efficacy and safety of the therapeutic in patients with moderate-to-severe psoriasis, a chronic immune-mediated disorder.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
InnoCare co-founder, chairwoman and CEO Dr Jasmine Cui said: “There are significant unmet needs for the treatment of autoimmune diseases such as psoriasis.
“InnoCare is developing autoimmune therapeutics with global frontier targets through B-cell and T-cell pathways, including orelabrutinib (BTK inhibitor), ICP-332 (TYK2-JH1 inhibitor), ICP-488 (TYK2-JH2 inhibitor), and more.
“We will accelerate clinical development of the innovative drugs, and look forward to providing better treatment options for patients with autoimmune diseases such as psoriasis.”
A well-tolerated safety profile of ICP-488 was demonstrated in a Phase I clinical trial, which involved the administration of single and multiple ascending doses to psoriasis patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataICP-488 binds the JH2 domain and blocks the signal transduction of interleukin (IL)-23, IL-12, type 1 IFN, and other cytokines.
It also has the potential to treat other autoimmune disorders, in addition to psoriasis.
Last December, InnoCare received approval from China’s Center for Drug Evaluation (CDE) to initiate a Phase II clinical trial of orelabrutinib, along with tafasitamab + lenalidomide, to treat relapsed or refractory Non-Hodgkin’s lymphoma.
The combination of tafasitamab and lenalidomide has been approved for treating patients with diffuse large B-Cell lymphoma.
The approval has been granted under the early access programme in the Boao Lecheng International Medical Tourism Pilot Zone by the Health Commission and Medical Products Administration of the Hainan Province.





