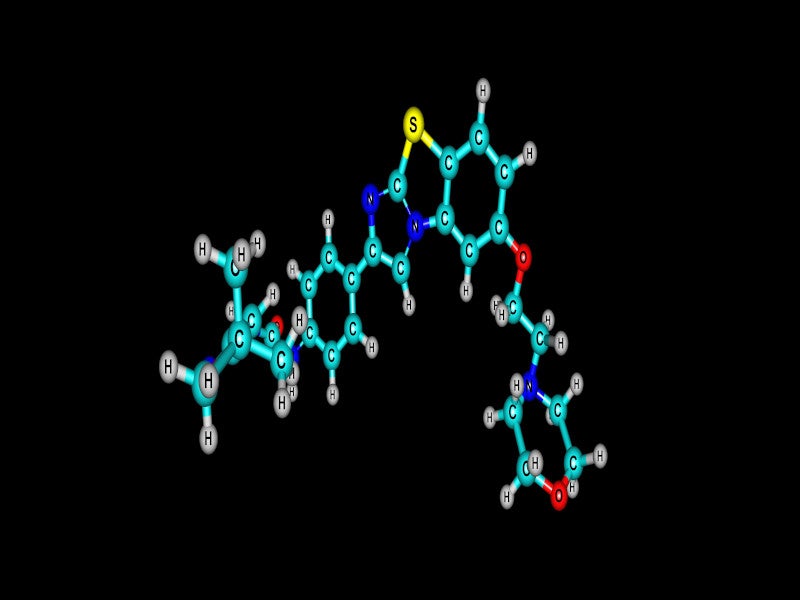
VANFLYTA® (quizartinib) is an oral kinase inhibitor indicated for the treatment of adult patients with newly diagnosed acute myeloid leukaemia (AML), that is FMS-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) positive as confirmed by a US Food and Drug Administration (FDA)-approved test.
VANFLYTA is approved to be taken in combination with standard cytarabine and anthracycline induction and cytarabine consolidation and as a maintenance monotherapy following consolidation chemotherapy for the condition.
It is the first FLT3 inhibitor to be approved by the FDA specifically for FLT3-ITD positive AML and across the three phases of treatment, including induction, consolidation and maintenance in patients without transplant for the condition.
VANFLYTA was developed by the Japanese pharmaceutical company Daiichi Sankyo. The company selected Biologics by McKesson as a speciality pharmacy provider for VANFLYTA in August 2023.
VANFLYTA is available as film-coated, round, oral tablets in white colour for 17.7mg dosage strength and yellow colour for 26.5mg strength.
Regulatory approvals for VANFLYTA
VANFLYTA received marketing approval from Japan’s Ministry of Health, Labour and Welfare (MHLW) as a monotherapy for relapsed/refractory FLT3-ITD in June 2019, followed by its launch in October 2019.
The drug was approved in Japan to treat patients with newly diagnosed AML, that is FLT3-ITD mutation-positive, in May 2023.
The US FDA approved VANFLYTA (quizartinib) in July 2023 to treat adult patients with FLT3-ITD positive AML. It has received priority review and fast-track designation for the indication from the FDA.
Invivoscribe Technologies’ LeukoStrat® CDx FLT3 Mutation Assay, a PCR-based in vitro diagnostic test, received FDA approval as a VANFLYTA companion diagnostic in July 2023 to identify FLT3-ITD positive AML patients who may be treated with VANFLYTA.
Acute myeloid leukaemia causes and symptoms
AML is a rare and aggressive cancer affecting blood and bone marrow, causing uncontrolled growth and accumulation of malignant white blood cells. FLT3-ITD is the most common mutation, affecting one in four patients with AML.
The newly diagnosed FLT3-ITD positive AML is one of the most aggressive and difficult-to-treat subtypes of AML.
Approximately 37% of newly diagnosed AML patients exhibit an FLT3 gene mutation, with approximately 80% of such cases involving FLT3-ITD mutations. The mutations are responsible for triggering cancer progression and are linked to an elevated risk of relapse and reduced overall survival.
It is caused by various factors including age, cancer therapies, radiation exposure, smoking, blood conditions, bone marrow abnormalities and genetic disorders.
AML causes persistent symptoms, including dizziness, easy bleeding, fatigue, fever, night sweats, infections, headaches, loss of appetite, weight loss, pale skin, shortness of breath, swollen lymph nodes and persistent wounds or sores.
AML is the most common adult leukaemia, accounting for 23.1% of total leukaemia cases globally. In Japan, 7,000 new cases are diagnosed annually, with a 21.1% five-year overall survival rate in adult patients.
VANFLYTA’s mechanism of action
VANFLYTA is a highly potent small molecule inhibitor of the receptor tyrosine kinase FLT3.
Quizartinib and its principal active metabolite AC886 bind to the ATP binding domain of FLT3 with comparable affinity and both have a ten-fold reduced affinity for the FLT3-ITD mutation compared to FLT3 in a binding assay.
Quizartinib and AC886 suppress FLT3 kinase activity, preventing receptor autophosphorylation, thus suppressing downstream FLT3 receptor signalling and blocking cell growth dependent on FLT3-ITD.
Clinical trials on VANFLYTA
The approval of VANFLYTA for newly diagnosed FLT3-ITD positive AML was supported by a randomised, double-blind, placebo-controlled global phase III clinical trial named QuANTUM-FIRST.
The study evaluated the drug in combination with standard induction and consolidation therapy, including haematopoietic stem cell transplantation (HSCT) and as maintenance monotherapy, in adult patients aged between 18 years and 75 years with newly diagnosed FLT3-ITD positive AML. Overall survival was the primary endpoint of the study.
The patients were randomised in a 1:1 ratio to receive either VANFLYTA or placebo combined with standard cytarabine, anthracycline induction and cytarabine consolidation and continued as maintenance monotherapy following consolidation.
The study results indicated a 22% reduction in the risk of death compared to standard chemotherapy alone in patients with newly diagnosed FLT3-ITD positive AML. The complete remission (CR) rates were similar in both the trial groups, while the median duration of CR was more than three times longer at 38.6 months for patients receiving VANFLYTA compared to 12.4 months for those receiving placebo plus standard chemotherapy alone.
Common adverse effects include febrile neutropenia, diarrhoea, mucositis, nausea, stomach discomfort, sepsis, headache, elevated creatine phosphokinase, vomiting and upper respiratory tract infection.
Additional trials on VANFLYTA
The approval of VANFLYTA in Japan as a monotherapy for relapsed/refractory FLT3-ITD AML was based on the results of the global pivotal phase III QuANTUM-R study and a single-arm phase II study evaluating VANFLYTA in Japanese patients with relapsed/refractory FLT3-ITD AML.
The trial showed a significant improvement in overall survival compared to salvage chemotherapy.
The most common treatment-related adverse effects included nausea, electrocardiogram QT prolonged, anaemia and thrombocytopenia.