Decentralised clinical trials (DCTs) have become synonymous with patient centricity. The concept of decentralisation is widely considered to be the epitome of a patient-focused approach to clinical trial design.
However, completely decentralised trials impose comparable constraints on trial participants compared to traditional clinical trials by removing their option to visit a clinical site.
At the 10th Annual Outsourcing in Clinical Trials (OCT) conference from 5-6 September 2023, Dr Claudia Hesselmann, founder and CEO of Arensia, reiterated that many trial participants do not want a clinical trial to invade their homes.
There are countless reasons for this preference.
Trial participants may not want neighbours or even their own families to know about their situation.
The physical and emotional journey of a trial can be painful, and many participants consider their home to be a place where they can escape those feelings, so having it become a reminder of what they are going through can be unsettling.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe responsibility of ensuring that participants are using their wearable and in-home devices correctly can also take a mental toll and become an added chore.
Many may also struggle with the reduction in face-to-face interactions with clinical professionals, which can provide immense reassurance and comfort.
Regardless of the reasons why someone may not want to participate in a DCT, an eligible participant should not be excluded for having specific preferences. True patient centricity is giving these participants a choice.
The term ‘patient centricity’ itself is flawed, as it reinforces the idea of trial participants as patients.
At the OCT conference, Dr Janet Messer, director of Approval Service, Health Research Authority, proposed that this term should be changed to ‘people centricity’, as the entire concept is to humanise the people within a clinical trial.
What they deserve, she added, is to be given the opportunity to influence clinical trial design to avoid assumptions being made about what they want.
Hybrid DCT designs have been widely used for decades. These trials may be more patient-centric than DCTs as they can provide participants with greater options.
Travelling to a site is not a major barrier for all participants, but being able to participate in aspects of the trial remotely may be necessary for these people at some points, especially throughout longer Phase III trials.
Similarly, some patients may want paper options for diaries while others prefer digital diaries.
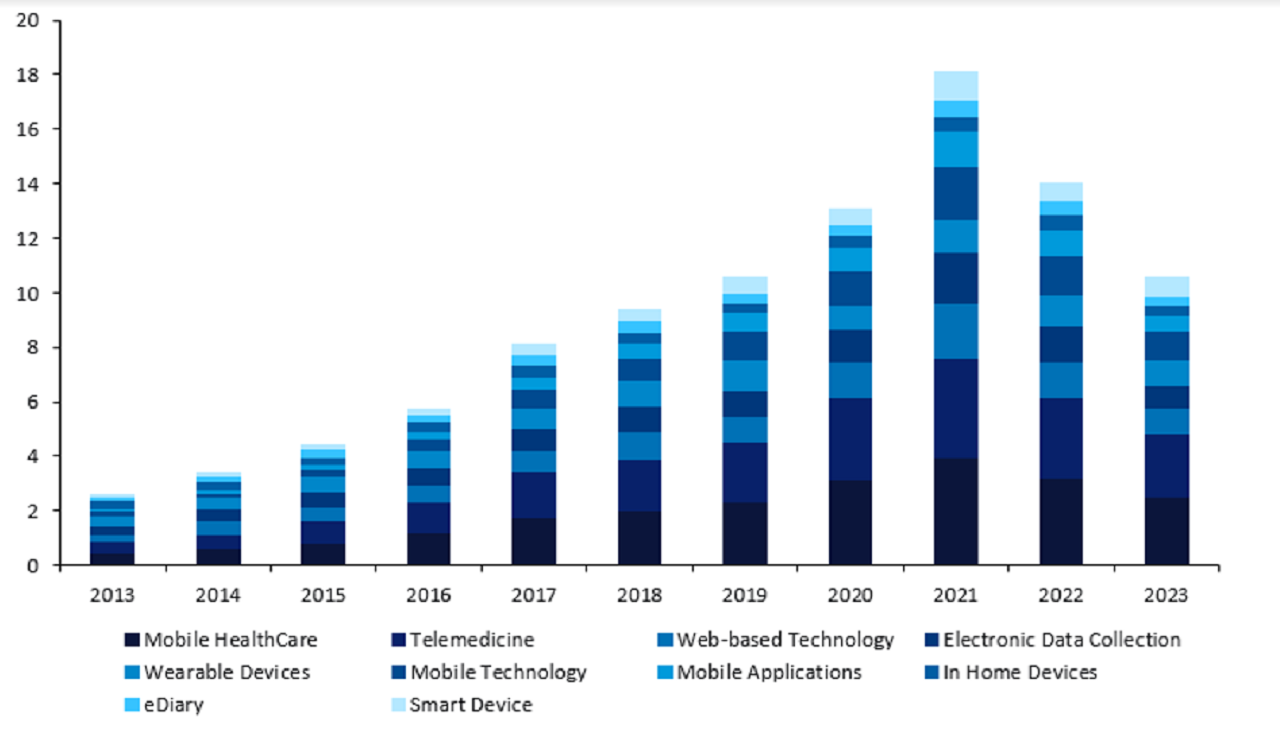
GlobalData’s Clinical Trials database shows that the use of virtual components has drastically increased over the last ten years (Figure 1).
Trials utilising virtual components peaked in 2021 following the Covid-19 pandemic, but 2022 saw figures remain higher than in pre-pandemic years, indicating that these approaches proved successful.
Although DCTs and hybrid DCTs are generally considered to be patient-centric trial designs while traditional trials are not, this is not the case.
Industry, sponsors, and sites are learning that they need to connect and align with patients to improve their experience, as the patients’ perspectives may differ from what is assumed.
Although companies running DCTs and focusing on patient centricity should be applauded, the perpetual issue of accrual and retention across trials indicates that there is still a long way to go before true people centricity is achieved throughout all clinical trials.