Transparency throughout the entire clinical trial industry was a persistent theme at the tenth annual Outsourcing in Clinical Trials DACH conference on 22-23 November. It started with Natalia Buchneva, risk management lead for clinical data and innovations at UCB, who detailed the revision of good clinical practice (GCP) guidelines, which comprise a set of internationally recognised ethical and scientific quality requirements that must be followed by everyone delivering a clinical trial. Buchneva explained that under new requirements set to launch in November 2024, all trials must be publicly announced, and results disclosed. She further detailed that sponsors will be required to share trial results with participants if requested; not only will this undoubtedly increase trial transparency, but returning participant data will enable them to be empowered in their healthcare.
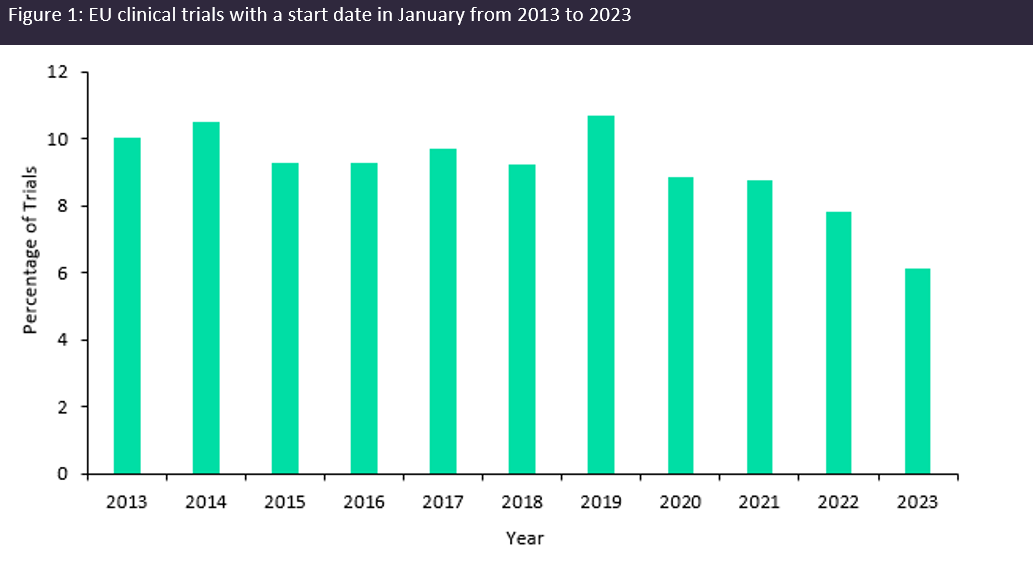
The theme of trial transparency being weaved into regulations was then echoed by Diana Filipescu, business development manager at EastHORN, when discussing the implementation of the clinical trial information system (CTIS), which became the single portal that sponsors and regulators must navigate to submit new clinical trial applications in the EU as of January 2023. The CTIS application overhaul has increased trial transparency as trial information is to be made publicly available. However, trepidations from sponsors initiating trials in Europe following these changes have been widely reported and the clinical trial landscape throughout Europe suffered as a result. Data obtained from GlobalData’s Trials Intelligence platform shows that the number of clinical trials initiated as CTIS was implemented in January hit a ten-year low (Figure 1, above). Therefore, it is reasonable to assume that there will be similar discourse globally as the launch date for the updated GCP guidelines draws nearer.
The conversation around transparency then pivoted and the benefits of trial transparency from the perspective of a participant were presented by Estelle Jobson, EUPATI Fellow and Patient Expert. Jobson proposed the potential effect on participant recruitment and retention by implementing a series of changes right from pre-study, such as including the participants within protocol design and treating them more as a “research partner” as she explained “what greater contribution is there than their own bodies”.
Lastly, the request for transparency from a sponsor’s point of view was presented by Seagen Europe senior director for regional clinical trial operations Edward Walsh, who expressed how transparency from contract research organisations (CROs) regarding trial timelines and costs will provide more harmony throughout a clinical trial.
All in all, it appears that the lack of transparency throughout several areas of the clinical trial industry has been recognised and regulations that will increase transparency are coming into effect. Hopefully, this movement towards trial transparency can ultimately improve clinical trials globally as well as participants’ experience, as this will fuel the number of innovative therapies available to address patients’ unmet needs.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData