Rezdiffra (resmetirom) is a novel therapeutic indicated alongside diet and exercise for the treatment of adults with noncirrhotic non-alcoholic steatohepatitis (NASH) with moderate to advanced liver fibrosis, corresponding to stages F2 to F3 fibrosis.
Developed by the US-based biopharmaceutical company Madrigal Pharmaceuticals, Rezdiffra acts as a thyroid hormone receptor-beta (THR-β) agonist, targeting the underlying causes of NASH.
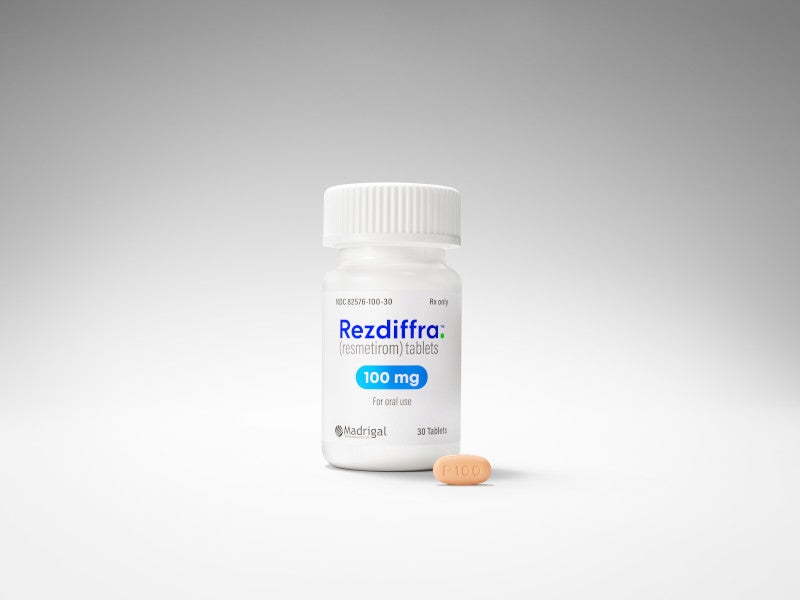
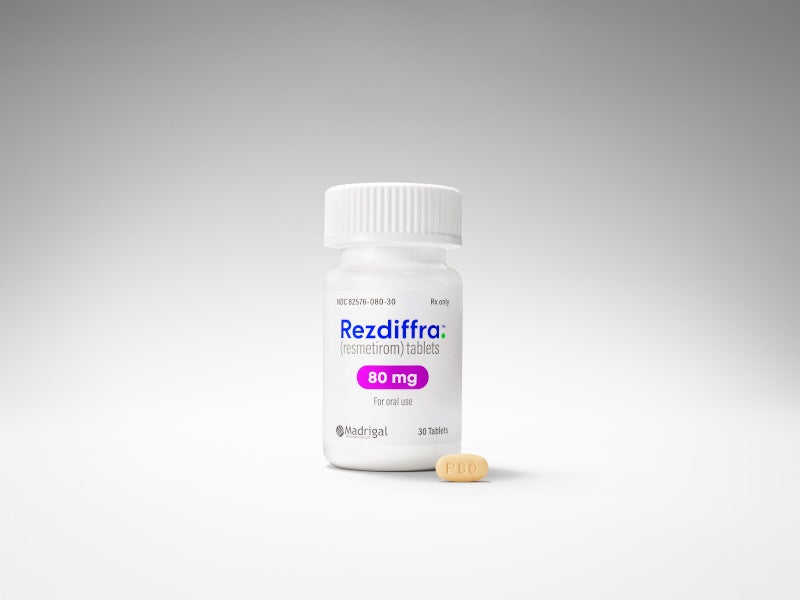
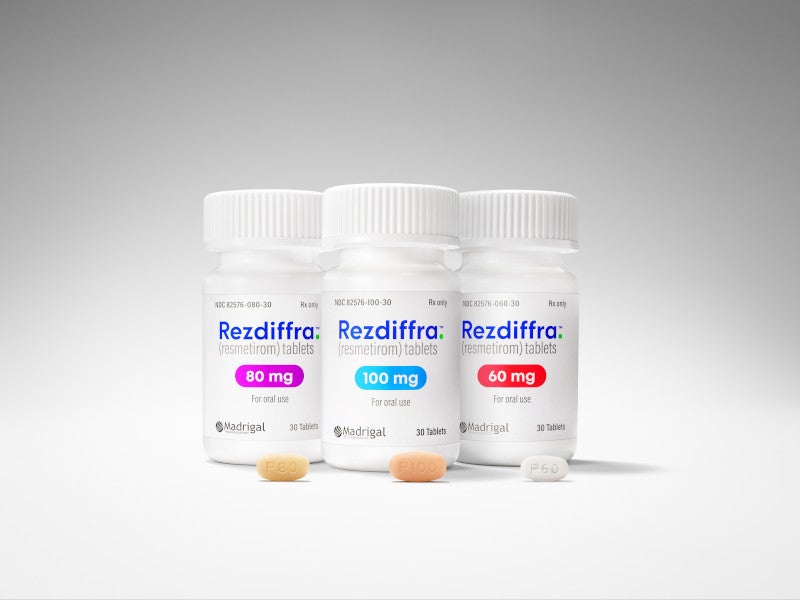
The drug is available as oval-shaped, film-coated tablets in three different dosage strengths, 60mg in white colour, 80mg in yellow colour, and 100mg in beige to pink colour.
Regulatory approvals for Rezdiffra
The US Food and Drug Administration (FDA) granted accelerated approval to Rezdiffra in March 2024, following the submission and acceptance of a new drug application in July and September 2023, respectively.
Rezdiffra has also received breakthrough therapy, fast track, and priority review designations in the US.
In Europe, the marketing authorisation application for Rezdiffra is currently under review by the European Medicines Agency Committee for Medicinal Products for Human Use.
NASH causes and symptoms
NASH, a severe form of non-alcoholic fatty liver disease (NAFLD), is caused by the accumulation of fat in the liver, which is not due to alcohol consumption. Symptoms may not be apparent in the early stages, but patients may experience fatigue, weakness, and abdominal pain as the disease progresses.
NASH poses significant health risks and is a growing concern for healthcare systems worldwide. It is a leading cause of liver-related mortality and is becoming the primary reason for liver transplants in the US.
The condition is characterised by hepatic steatosis, inflammation, and varying degrees of fibrosis, potentially leading to cirrhosis and hepatocellular carcinoma.
It is often associated with metabolic co-morbidities such as obesity, type 2 diabetes, hypertension, and dyslipidaemia. As the prevalence of the metabolic risk factors rises, so does the incidence of NASH, making it a pressing health concern.
The disease’s progression to stages F2 to F3 fibrosis significantly raises the likelihood of adverse liver outcomes.
Rezdiffra’s mechanism of action
Rezdiffra contains resmetirom, a partial agonist for the THR-β. The receptor subtype is predominantly found in the liver, where it plays a significant role in lipid metabolism.
Activation of THR-β reduces intrahepatic triglycerides, while the effects of thyroid hormone on the heart and bone are primarily mediated through THR-α. Resmetirom has demonstrated selective efficacy in activating THR-β over THR-α in vitro assays.
The drug has been shown to produce 83.8% of the maximum response when compared to triiodothyronine, the natural thyroid hormone, with an effective concentration of 0.21 µM within in vitro assays.
Clinical trials on Rezdiffra
The accelerated FDA approval of Rezdiffra was based on the ongoing Phase III MAESTRO-NASH trial. It is an ongoing pivotal, multicentre, randomised, double-blind, placebo-controlled study, which enrolled 1,759 patients with biopsy-confirmed NASH.
Participants were randomised in 1:1:1 ratio to receive either 80mg or 100mg of Rezdiffra or a placebo, once daily. The trial’s primary endpoints at 52 weeks were NASH resolution without fibrosis worsening and fibrosis improvement without NAFLD activity score worsening.
After 52 weeks, both doses of Rezdiffra showed significant improvements and a key secondary endpoint, the percent change from baseline in low-density lipoprotein cholesterol at week 24. The drug also improved liver enzymes, fibrosis biomarkers, and imaging test results compared to placebo.
The most reported adverse reactions in patients treated with Rezdiffra included diarrhoea, nausea, pruritis, abdominal pain, vomiting, constipation, and dizziness.
A distinct non-invasive Phase III trial, MAESTRO-NAFLD-1, was conducted to assess the safety and tolerance of Rezdiffra. The trial also added valuable data to the safety database used in regulatory evaluations of benefit-risk ratios.