The treatment of the hepatitis C virus (HCV) has been a controversial subject of health policy in the US, spurring multiple state policy shifts and retaliatory lawsuits since the introduction of effective antiviral therapies.
The fractious landscape of HCV treatment has been exemplified in state Medicaid coverage policies, which widely vary in their restrictions on patients’ access to care.
In an April 2024 publication in the JAMA Health Forum journal, Davey and colleagues conducted the first comprehensive analysis of state Medicaid coverage policies on HCV antiviral use.
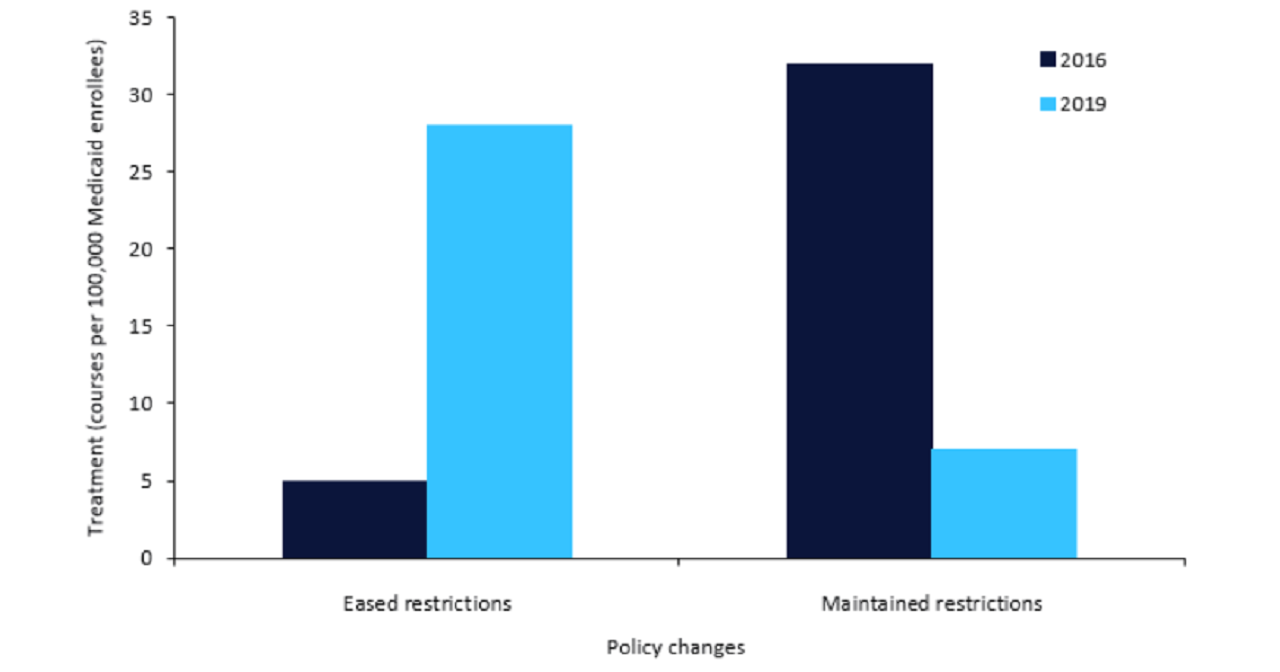
Results indicated that in states that eased restrictions on direct antiviral coverage, treatment use significantly increased when compared to states that maintained or strengthened restrictions.
GlobalData epidemiologists forecast that in the US, the diagnosed prevalent cases positive for HCV RNA increased from more than 867,200 cases to approximately 882,400 cases between 2015 and 2025.
The growing burden of HCV emphasises the need for a coherent policy towards HCV antivirals that allow for adequate care access.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn their analysis, Davey and colleagues considered 39 states’ Medicaid policies from 2015, which was shortly after the first HCV direct antiviral combination therapy’s introduction, to 2019.
Policies were categorised as having strict, lenient, or unrestricted coverage.
While states with strict coverage were defined as those only covering treatment of patients with F3 or F4 fibrosis scores, states with lenient coverage conditioned treatment on clinical consultations and patient sobriety adherence.
To estimate the number of antiviral therapies administered, the authors leveraged publicly available Medicaid drug utilisation and enrolment data.
Among all analysed states, 25 eased their coverage policies, three eliminated restrictions, and seven did not change their policies.
As displayed in Figure 1, between 2016 and 2019, states that eased or ended restrictions saw an increase from five HCV treatment courses per 100,000 Medicaid enrollees to 28 HCV treatment courses per 100,000 Medicaid enrollees at the end of the study period.
However, the results also indicated lingering disparities in treatment.
When compared with coverage provided by managed care organisations (MCOs), only Medicaid beneficiaries saw an increase in HCV antiviral use.
Additionally, the easing of sobriety restrictions in particular was not correlated with increased therapy use.
The authors attribute these exceptions, respectively, to deficiencies in states’ compliance enforcement toward MCOs and reticence among substance users to seek treatment due to stigma among healthcare providers.
The findings presented in the analysis by Davey and colleagues present a stark contrast in health policies targeting HCV treatment.
While the proliferation of diverse antiviral treatments has driven down costs while covering a wider range of HCV genotypes, access to care remains highly inconsistent for the disadvantaged population depending on the policies in their states of residence.
Accordingly, Davey and colleagues propose a more coherent standard for HCV Medicare coverage guidelines by lifting restrictions on direct antiviral treatment.
The steady pullback of rules on sobriety and fibrosis staging rules suggests a trend in this direction.
However, the heterogeneous nature of state Medicaid policies, coupled with wider institutional and sociocultural barriers to expanded coverage, present a formidable challenge to wider treatment options for HCV patients.