ANKTIVA® (nogapendekin alfa inbakicept), in combination with bacillus Calmette-Guerin (BCG) – a new treatment for non-muscle invasive bladder cancer (NMIBC), is indicated for adult patients with BCG-unresponsive NMIBC, including carcinoma in situ (CIS), with or without papillary tumours.
Developed by US-based biotechnology company ImmunityBio, ANKTIVA is the first-in-class interleukin-15 (IL-15) receptor agonist targeting BCG-unresponsive NMIBC. ANKTIVA enables a targeted attack on tumour cells by activating the body’s natural killer and killer T-cell immune system.
It is an advanced immunotherapy beyond checkpoint inhibitors, serving as an alternative to radical cystectomy, a surgical procedure for patients having limited options after the failure of BCG.
ANKTIVA is administered as a clear-to-slightly opalescent, colourless-to-slightly-yellow solution in a 400mcg/0.4ml strength, provided in single-dose vials for intravesical instillation after dilution.
The US Food and Drug Administration (FDA) approved ANKTIVA based on the positive results from the QUILT 3.032 clinical study, in April 2024.
ImmunityBio signed an exclusive worldwide deal with pharmaceutical company the Serum Institute of India in May 2024, to provide BCG. The collaboration will lead to the large-scale production of BCG for use alongside ANKTIVA.
The agreement involves producing standard BCG (sBCG), which is currently authorised for use outside the US, and a next-gen recombinant BCG (iBCG) still undergoing trials. iBCG is intended for use alongside ANKTIVA for both current and potential future applications, pending regulatory clearance.
Bladder cancer causes and symptoms
Bladder cancer originates in the urothelial cells lining the bladder and can also affect the kidneys and ureters. Symptoms such as blood in the urine (haematuria), frequent urination, painful urination and back pain can signal the presence of the disease. While early-stage bladder cancers are highly treatable, the risk of recurrence necessitates long-term follow-up tests.
Globally, bladder cancer ranks as the tenth most diagnosed cancer. The American Cancer Society estimates that the US alone will witness 83,190 new cases and 16,840 deaths due to bladder cancer in 2024.
The typical treatment for NMIBC involves delivering BCG directly into the bladder through a catheter. BCG, a harmless bacterium, triggers an immune reaction in the bladder near the cancerous cells, often resulting in the eradication of the cancer. But BCG proves ineffective in about 30% to 40% of cases, and approximately 50% of those who initially respond will experience a recurrence.
ANKTIVA’s mechanism of action
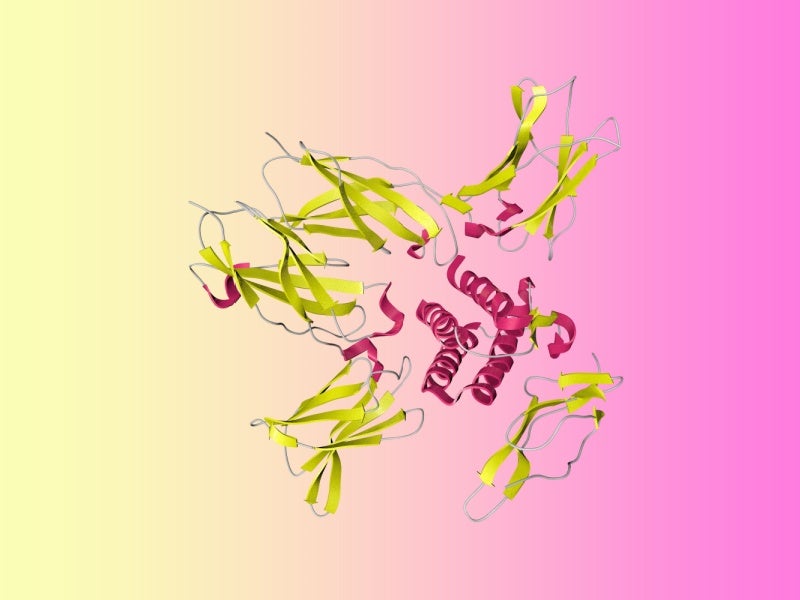
The cytokine IL-15 is pivotal in the immune system, influencing the development, upkeep and functionality of crucial immune cells, such as NK and CD8+ killer T cells, which play a role in eliminating cancer cells.
ANKTIVA represents a new IL-15 superagonist complex featuring an IL-15 mutant (IL-15N72D) fused with an IL-15 receptor alpha. The complex binds strongly to IL-15 receptors on NK, CD4 and CD8 T cells, akin to the natural actions of dendritic cells. Consequently, it fosters the creation of memory-killer T cells that are specifically primed to target cancer cells.
Subsequently, the activation and proliferation of these cytotoxic cells results in a sustained complete response. ANKTIVA® features enhanced pharmacokinetic characteristics, prolonged presence in lymphoid tissues, and heightened anti-tumour efficacy in comparison to native, un-complexed IL-15 in vivo.
Clinical trials on ANKTIVA
The FDA’s approval of ANKTIVA® is based on the safety and efficacy data from the single-arm, multi-centre, Phase II/III QUILT 3.032 clinical trial.
A total of 77 evaluable patients with high-risk, BCG-unresponsive NMIBC with CIS, with or without Ta/T1 papillary disease, following transurethral resection, received ANKTIVA® with BCG maintenance therapy for up to 37 months.
Tumour status was rigorously assessed through cystoscopy, urine cytology every three months for two years, and mandatory biopsy within six months of treatment initiation.
The primary efficacy measures were the complete response (CR) rate and the duration of complete response (DOR). The trial reported a CR rate of 62% for the evaluable patients.
The DOR was more than 47 months, as of November 2023, and is still ongoing. The extended period of response, surpassing 47 months, is a significant breakthrough for NMIBC patients. It offers additional clinical proof of ANKTIVA’s effectiveness for patients who typically experience frequent recurrence and a considerable decline in their quality of life due to radical surgeries.
Among patients who achieved CR, 58% had a response lasting at least 12 months, and 40% had a response lasting at least 24 months.
The most common adverse reactions observed were increased creatinine, dysuria, haematuria, urinary frequency, micturition urgency, urinary tract infection, increased potassium, musculoskeletal pain, chills and pyrexia.