Adoptive cell therapy is a type of immunotherapy comprising cells that are engineered and expanded ex vivo and administered to stimulate the immune system and kill tumour cells or other diseased cells. There are three main categories of adoptive cell therapies: tumour-infiltrating lymphocyte (TIL) immunotherapy, natural killer (NK) cell immunotherapy, and T cell immunotherapy. The most popular type of adoptive cell therapy by far is T cell immunotherapy, specifically chimeric antigen receptor (CAR)-T cell therapy, which accounts for 55% of all adoptive cell therapy trials, according to data obtained from GlobalData’s Trials Intelligence platform.
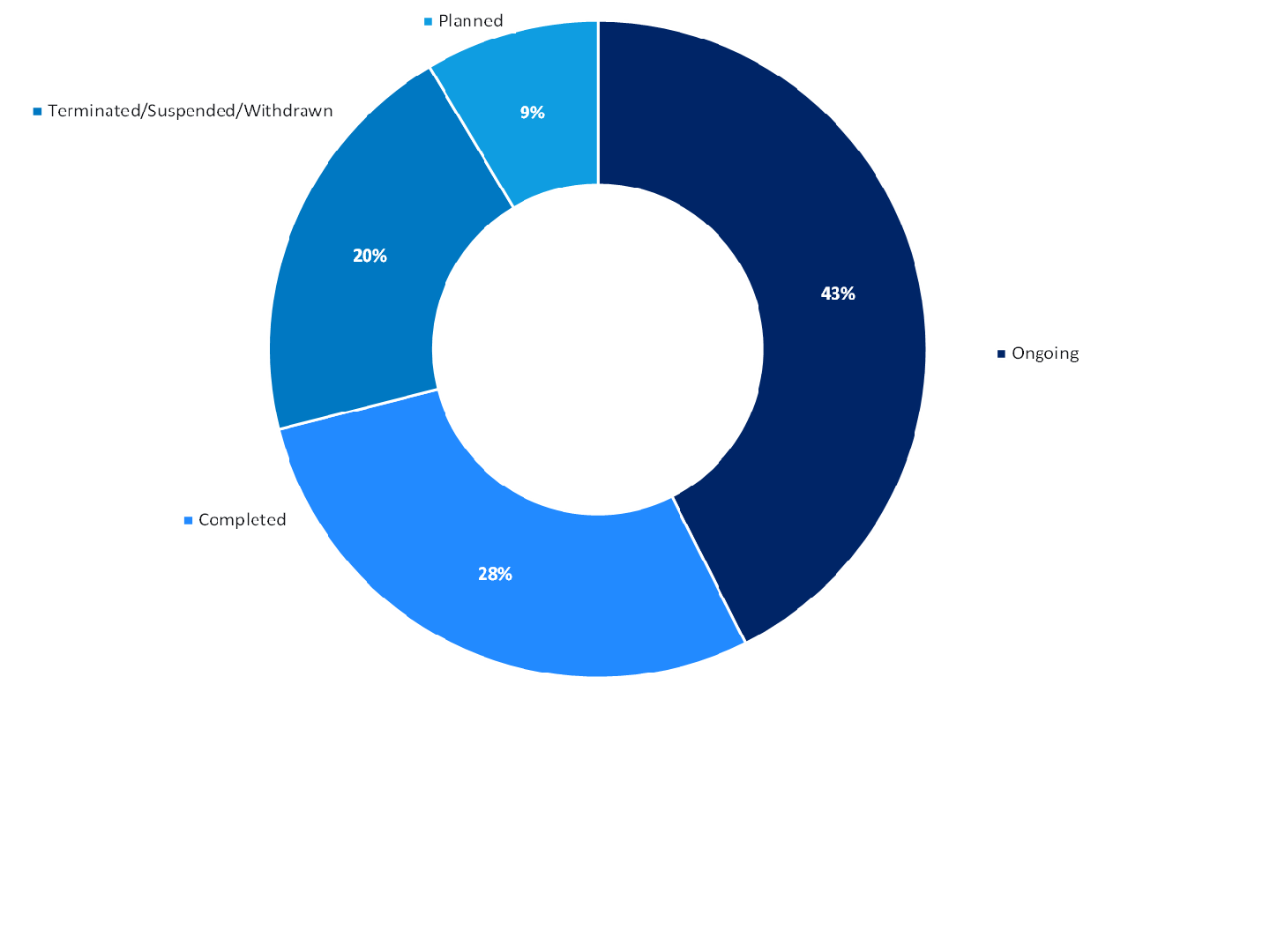
Adoptive cell therapy is still in its infancy however, additional insights from GlobalData’s Trials Intelligence platform show that around 42% of all adoptive cell therapy trials are ongoing (Figure 1, above), indicating that there may be an upcoming influx of adoptive cell therapies in the pipeline. However, most of these trials are in early phases so there is a long road ahead to see if these promising therapies can progress to the market.
Furthermore, there is a significantly high proportion of adoptive cell therapy trials that have either been suspended, terminated, or withdrawn (Figure 1), indicating the presence of added safety and efficacy concerns associated with biological factors and the increased difficulty with advancing these therapies. Although the number of ongoing studies demonstrate some sponsors are tackling these difficulties head on, it has only been 12 years since the first paediatric patient received CAR-T therapy and seven years since the first CAR-T therapies were commercialised so only time will determine how the landscape of adoptive cell therapy will change.