VenoStent, a Texas based medical device company, has raised $20m in Series A financing to help develop its bioabsorbable perivascular wrap, SelfWrap.
The Series A investors included US-based venture capital firms Norwest Venture Partners, Good Growth Capital and IAG Capital Partners. The company also received a $3.6m Small Business Innovation Research (SBIR) Phase II Grant from the US National Institutes of Health (NIH).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
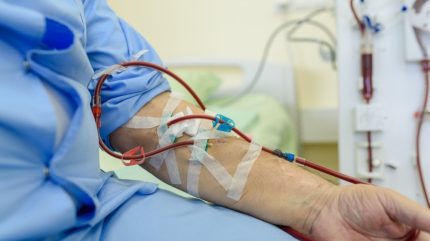
SelfWrap is designed to improve the usability and durability of arteriovenous fistulas (AVFs) created to enable haemodialysis treatment in patients with chronic kidney disease (CKD). AVFs are made by joining a vein onto an artery, this makes the vein grow stronger and allow for more blood to flow through it. Thereby, making it possible for dialysis nurses to repeatedly insert the needles needed for haemodialysis treatment.
The NIH grant will fund the registrational randomised control trial (NCT06001827) for the SelfWrap device. The SAVE-FistulaS (the SelfWrap-Assisted ArterioVEnous Fistula Study) is expected to enrol 200 patients expected to undergo a vascular access surgery.
The participants will either receive treatment with SelfWrap or the untreated AVF control and will be followed for 36 months. The primary endpoints of the trial are unassisted access for up to six months, and freedom from access-related adverse events through 30 days.
“Over half a million people in the US rely on haemodialysis to survive and require an arteriovenous fistula creation surgery in order to receive the treatment. However, the AV fistula procedure has a one-year failure rate of more than 60%, which significantly impacts patients’ survival rates and quality of life,” said Norwest General Partner and surgeon Dr Zack Scott.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“VenoStent’s groundbreaking technology for AV fistula formation, SelfWrap, has the potential to significantly improve these odds. We look forward to working with the VenoStent team as it proves the efficacy of this breakthrough technology in order to improve the lives of hundreds of thousands of CKD patients.”
The vascular access device for haemodialysis market is forecast to be worth $936.6m in 2030, as per GlobalData analysis.
GlobalData is the parent company of Clinical Trials Arena.
Xeltis is also developing a dialysis access device, aXess vascular graft. The device was was developed usingthe company’s proprietary endogenous tissue restoration (ETR) platform that utilises biodegradable polymer. It dissolves over time and creates a new, long-term living vessel for haemodialysis vascular access.
Another device in the AVF space is US Food and Drug Administration (FDA) approved VasQ External Vascular Support device. The Laminate Medical Technologies’ device can be used to create ACFs for dialysis access.





