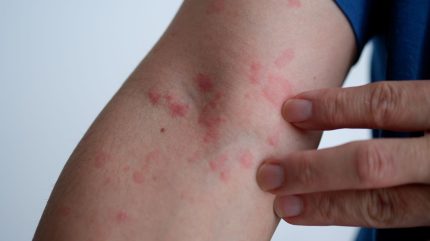
Evommune has enrolled the first subject in a Phase II clinical trial of EVO756 designed to treat adults with chronic inducible urticaria (CIndU).
This multicentre study aims to assess the safety and efficacy of EVO756 in approximately 30 patients suffering from symptomatic dermographism or cold urticaria, which are the two most prevalent types of CIndU.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It will use disease-specific provocation thresholds to measure changes from baseline, providing objective data on disease severity and treatment response.
Variation from baseline in disease-specific provocation thresholds used as objective indicators to assess disease severity and treatment response are the trial’s efficacy endpoints.
Patients will receive EVO756 orally, once a day for four weeks, with their condition monitored through weekly visits.
The trial has also deployed an approach that allows patients to serve as their own control.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataEVO756 is a selective Mas-related G protein-coupled receptor X2 (MRGPRX2) antagonist and could offer a new oral treatment option for mast cell-mediated diseases, with pre-clinical data claimed to indicate its ability to prevent mast cell degranulation across all relevant ligand categories.
The asset is also said to have the potential to rapidly alleviate itching associated with inflammatory diseases such as atopic dermatitis, due to its unique action on peripheral sensory neurons.
CIndU can lead to sleep deprivation, anxiety, depression, and social isolation, severely impacting patients’ quality of life.
Evommune clinical development vice-president J Mark Jackson said: “As we continue to execute on our clinical development plans for EVO756, we are commencing this Phase II trial in 15 study sites across the United States.
“CIndU patients have limited treatment options and we believe a novel, highly potent selective agent, which can be orally administered once daily, could provide patients with major therapeutic benefit.”
In January this year, Evommune commenced a Phase I first-in-human clinical trial of new oral treatment, EVO756, for chronic spontaneous urticaria (CSU).





