VYLOY™ (zolbetuximab) is an anti-claudin (CLDN)-18.2 monoclonal antibody used in conjunction with chemotherapy for the treatment of patients with human epidermal growth factor receptor 2 (HER2)-negative and CLDN18.2 positive, unresectable, advanced or recurrent gastric cancer.
Zolbetuximab, also known as claudiximab, was originally part of the drug pipeline of Ganymed Pharmaceuticals, a biopharmaceutical company based in Germany.
Astellas Pharma, a pharmaceutical company based in Japan, acquired Ganymed Pharmaceuticals and added zolbetuximab to its oncology product pipeline in December 2016.
Astellas Pharma developed the VENTANA® CLDN18 (43-14A) RxDx Assay, an immunohistochemistry companion diagnostic test, in collaboration with Roche Diagnostics. The test is approved to identify patients who may benefit from VYLOY treatment.
VYLOY is administered intravenously through a drip into a vein for at least two hours in a hospital or clinic, under the supervision of a doctor specialising in cancer treatment.
Regulatory approvals for VYLOY
The Ministry of Health, Labour, and Welfare (MHLW) of Japan approved VYLOY (zolbetuximab) for the treatment of gastric cancer in March 2024. The drug became the first and only CLDN18.2-targeted treatment to receive approval from any regulatory body globally.
In August 2024, the UK’s Medicines and Healthcare Products Regulatory Agency approved VYLOY for the treatment of gastric cancer
The Center for Drug Evaluation of the China National Medical Products Administration accepted the biologics license application (BLA) for zolbetuximab in July 2023.
During the same month, the European Medicines Agency accepted a marketing authorisation application for zolbetuximab for review, while the US Food and Drug Administration (FDA) granted priority review for a BLA for zolbetuximab.
The BLA for zolbetuximab was resubmitted in May 2024, after the FDA issued a complete response letter in January 2024, due to third-party manufacturing deficiencies found during the facility’s pre-licence inspection. A decision on the application is expected in November 2024.
The drug is also being reviewed in Australia and several other countries.
Gastric cancer causes and symptoms
Gastric cancer, also known as stomach cancer, gastro-oesophageal junction cancer, or gastric adenocarcinoma, usually arises when cancerous cells begin proliferating within the stomach’s lining, particularly at the juncture where the oesophagus meets the stomach.
Gastric cancer ranks as the fifth most frequently diagnosed cancer globally and is the third most frequently occurring fatal cancer in Japan.
A variety of risk factors are linked to gastric cancer, including advanced age, a family history of the disease, certain infections, and smoking.
Gastric cancer may not exhibit any symptoms in its early stages, however, as the tumour enlarges, symptoms such as indigestion, stomach discomfort, a sensation of bloating after meals, mild nausea, a loss of appetite, heartburn, blood in the stool, vomiting, unintentional weight loss, jaundice,and ascites [a build-up of fluid in the space between the lining of the abdomen and abdominal organs] appear.
VYLOY’s mechanism of action
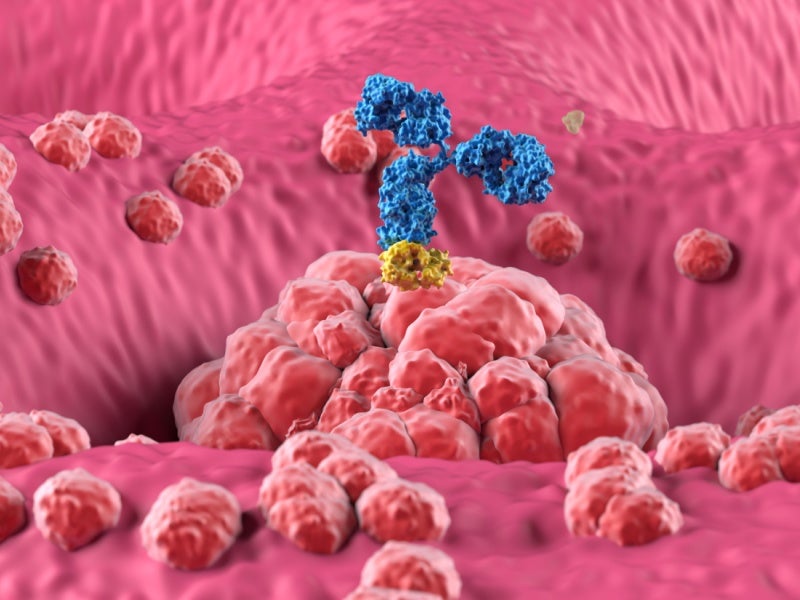
Zolbetuximab is a chimeric immunoglobulin G1 (IgG1) monoclonal antibody designed to target Claudin 18.2 (CLDN18.2), a transmembrane protein found in certain cancer cells, such as those in gastric and gastroesophageal cancers.
Claudin 18.2 is normally present in the stomach lining, but when cancer develops, it becomes exposed on the surface of the cancer cells.
Once zolbetuximab is injected into the body, it binds to Claudin 18.2 on the cancer cell surface, leading to the destruction of these CLDN18.2-expressing cancer cells by triggering the immune system through mechanisms called antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity, which stop the cancer cells from multiplying.
Clinical trials of VYLOY
The approval of VYLOY was based on the affirmative outcomes from two pivotal clinical trials SPOTLIGHT and GLOW.
SPOTLIGHT is a Phase III, international, multi-centre, double-blind, randomised trial, evaluating the efficacy and safety of VYLOY in combination with mFOLFOX6, a chemotherapy regimen comprising oxaliplatin, leucovorin and fluorouracil, against placebo plus mFOLFOX6 as a first-line treatment for patients with locally advanced inoperable or metastatic HER2-negative GAC exhibiting CLDN18.2 positivity.
The primary endpoint of the study was progression-free survival (PFS). Zolbetuximab plus mFOLFOX6 showed statistically significant improvements in PFS and overall survival (OS) compared to placebo plus mFOLFOX6.
Among the 565 patients recruited in the SPOTLIGHT trial, the cohort treated with VYLOY plus mFOLFOX6 demonstrated a 24.9% reduction in the chance of progression or death compared to the placebo. The median PFS reached 10.61 months in the treatment cohort and 8.67 months in the placebo cohort.
Furthermore, VYLOY plus mFOLFOX6 notably extended OS, condensing the mortality threat by 25%.
GLOW is a Phase III, international, multi-centre, double-blind, randomised trial to evaluate the efficacy and safety of VYLOY combined with the chemotherapy regimen including capecitabine and oxaliplatin (CAPOX) versus the placebo with CAPOX as a first-line treatment for patients with the specified condition.
The trial recruited 507 patients, and the primary endpoint of PFS was achieved with VYLOY in combination with CAPOX, which lessened the risk of progression or demise by 31.3%, with a median PFS of 8.21 months compared to the placebo group achieving a median PFS of 6.8 months.
Additionally, VYLOY plus CAPOX fulfilled the secondary endpoint of OS by evidencing a substantial decrease in mortality risk by 22.9% versus the placebo.
The most frequent adverse events observed in the patients included nausea, vomiting, reduced appetite, neutropenia and weight loss.