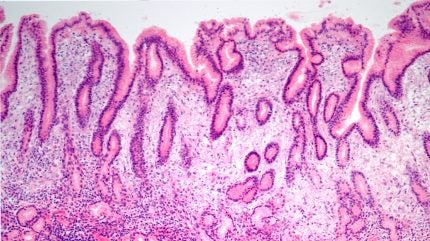
TenNor Therapeutics has announced a Phase III trial of its antibiotic candidate, rifasutenizol, has met all primary endpoints, beating out the current standard of care (SoC).
The multicentre, randomised, double-blind, controlled trial (NCT05857163) found that rifasutenizol triple therapy achieved >90% eradication rate, higher than bismuth-containing quadruple therapy (BQT), control in treating Helicobacter pylori (H. pylori) infection, a common bacterial infection of the stomach which can be a common cause of stomach ulcers.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
At the same time, the rifasutenizol regimen showed a better safety and tolerability profile than BQT. Only 3% of patients dropped out from the trial additionally seeing no significant differences in demographics or baseline antibiotic resistance characteristics between the two treatment groups.
Patients in the clarithromycin, metronidazole, levofloxacin and amoxicillin arms saw resistance rates of 41%, 68%, 35% and 8% respectively. The company says this is consistent with other reports and indicates an increase in anti-microbial resistance among strains of H. pylori across China. Antibiotic resistance is becoming a global issue, with more antibiotics and alternative therapies needed.
Rifasutenizol targets anaerobic and microaerophile bacteria and the Chinese-based company believes it could have the potential to become the first new drug developed specifically for H. pylori infection in more than 30 years.
Previously the company completed seven in-human clinical trials across China and has received Qualified Infectious Disease Product (QIDP) and fast track designations from the US Food and Drug Administration (FDA).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn the field of antibiotic resistance and drugs designed to mitigate its effects, Spero Therapeutics has suspended the development of its antibiotic SPR720, after the therapy failed to meet the primary endpoint. Meanwhile, Inflammatix has kick-started a clinical trial evaluating its technology for the management of patients with suspected acute infection.





