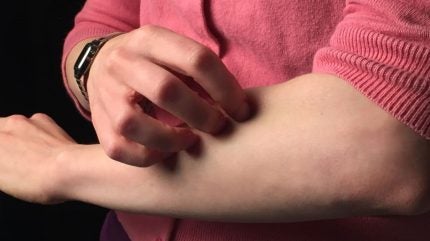
GSK has announced positive headline results of its ongoing global Phase III clinical trial GLISTEN, which is evaluating the use of linerixibat in the treatment of adult patients with cholestatic pruritus (relentless itch).
Cholestatic pruritus is a common symptom of primary biliary cholangitis (PBC), a rare autoimmune liver disease. Researchers are looking to establish the safety and efficacy of linerixibat – an investigational targeted inhibitor of the ileal bile acid transporter (IBAT) – for the treatment of cholestatic pruritus in PBC patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The results show that GLISTEN met its primary endpoint, and patients receiving linerixibat experienced a statistically significant improvement in itch in comparison to patients receiving a placebo.
The phase III linerixibat trial was made up of patients experiencing moderate to severe itch who were either receiving stable doses of guideline-suggested therapies for cholestatic pruritus, were treatment naïve or had been previously treated. It measured a monthly itch score over 24 weeks and compared the results to a baseline.
GLISTEN followed the phase IIb GLIMMER trial in 2020, which demonstrated that linerixibat significantly improved symptoms in some treatment groups compared to placebo.
Preliminary safety results were reported to be generally consistent with results seen in previous studies of linerixibat, although GSK said that further analysis of data will continue.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataKaivan Khavandi, SVP and global head of respiratory/immunology R&D at GSK, said: “Linerixibat has the potential to be the first global therapy specifically developed to treat itch in PBC. These positive data suggest that it could have a place in supporting patients whose quality of life is significantly affected in multiple ways by persistent itching.”
It is expected that PBC diagnoses will reach 510,000 globally by 2030, with women being disproportionately affected. The PBC foundation reports that the condition affects nine women for every man and notes that the most common age for diagnosis is between 35 and 55 years. Of the 510,000 PBC patients, 240,000 will experience cholestatic pruritus.
The FDA granted linerixibat orphan drug designation in the treatment of PBC and associated cholestatic pruritus in 2019. Administered as a tablet, the drug blocks the resorption of bile acids in the small intestine, reducing pruritic bile acids in circulation.
PBCers Organization president Carol Roberts said: “The itch associated with PBC for many patients is unrelenting and often severe but is a symptom that is frequently overlooked or dismissed. It has a significant impact on the quality of life and mental health of people with PBC. The potential of a treatment option that addresses a root cause of itch answers a previously unmet need for people with PBC.”
GSK will present the full results of GLISTEN at a future, currently unnamed scientific congress.





