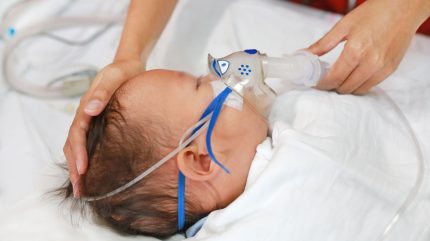
US-based biotechnology company Enanta Pharmaceuticals has reported positive topline outcomes from its randomised Phase II trial of zelicapavir, a potential treatment for respiratory syncytial virus (RSV) in paediatric patients.
The study enrolled hospitalised and non-hospitalised children aged from 28 days to 36 months, observing antiviral effects.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Of the 96 subjects enrolled, 70 received zelicapavir while 26 were given placebo.
Baseline characteristics and demographics were consistent across treatment groups, while the majority of subjects were hospitalised upon enrolment.
A subset of subjects who started treatment within three days of symptom onset experienced a 1.2 log decline in viral load, indicating a rapid antiviral response.
Zelicapavir also achieved the targeted drug exposure levels across all age groups and dosing cohorts.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSubjects aged 28 days to 12 months were given 5mg/kg of zelicapavir, while those aged 12-36 months each received a 7.5mg/kg dose.
Several methods, including the REspiratory Syncytial VIrus NETwork (ReSViNET) and the RESpiratory ObservabLE Reported Outcome-Paediatric (RESOLVE-P) tools, were used to assess symptoms due to the lack of validated symptom tools for paediatric RSV.
While ReSViNET showed no difference in symptoms between the treatment and placebo groups, the RESOLVE-P tool indicated a trend towards greater symptom reduction in the therapy-treated subjects.
Zelicapavir was well-tolerated in the trial, with a favourable safety profile compared to that of placebo.
Enanta Pharmaceuticals chief medical officer Scott Rottinghaus said: “We are excited to share these positive results from our first-in-paediatric Phase II study of zelicapavir, which we believe confirm a strong profile for our lead RSV antiviral and strengthen Enanta’s position as a leader in developing treatments for RSV.
“Zelicapavir demonstrated an antiviral effect on both primary endpoints, as well as secondary virology endpoints.”
Based in Massachusetts, Enanta Pharmaceuticals develops small-molecule drugs with an emphasis on virology and immunology indications.





