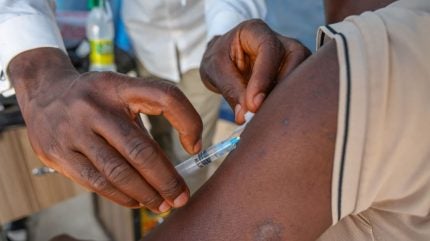
The Coalition for Epidemic Preparedness Innovations (CEPI) has announced that it is providing up to $9.9m for a vaccine safety project in Africa, led by the Global Vaccine Data Network (GVDN).
The Background Rates of Adverse Events for Vaccine Evaluation in Africa project will look to understand the incidence rates of naturally occurring clinical events within African populations such as Guillain-Barré Syndrome and sensorineural hearing loss.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
By developing the baseline data, the GVDN hopes to offer additional insight into whether an adverse event (AE) reported in a vaccine trial may be associated with the administered vaccine – an adverse event of special interest (AESI) – or whether the event is in line with the expected levels within the demographic.
CEPI’s clinical development director Jakob Cramer explained: “Having this newly formed network in place will help contextualise any adverse events reported in clinical trials and will ultimately enable timely evidence generation of vaccine safety during public health responses in Africa.”
The research consortium hopes that the findings will be considered by regulatory bodies to enable better-informed decisions about vaccine safety in African populations. A better understanding of AEs could also boost public confidence and tackle scepticism.
The project will use a platform previously developed by Gavi, the Vaccine Alliance, which conducted the active vaccine safety surveillance study in Africa during the Covid-19 pandemic, to estimate the risk of AESI. At its commencement, it will apply the data on the background rate of AEs to Lassa vaccines currently being developed by CEPI.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLed by the GVDN, the project is being run in partnership with the University of the Witwatersrand in South Africa, Navrongo Health Research Center in Ghana, International Foundation Against Infectious Diseases in Nigeria, Eagle Research Center in Rwanda and Health Research Operations Kenya (operating as KEMRI-Wellcome Trust Research Programme) in Kenya.
GVDN co-director Dr Steven Black said: “With this collaboration, the GVDN will be helping provide data to assure vaccines used in Africa are both safe and effective. With new pathogens emerging in this region, developing this capacity is critical.”
Despite evidence that vaccine responses differ across demographics, most vaccine safety infrastructure in African countries has been developed based on AE data generated in the Global North. A lack of specific data is an issue exacerbated by a complete absence of vaccine safety data for diseases endemic to Africa, for which vaccines are developed and introduced directly to affected countries.
There has been a recent push for diversity in the clinical trial space, with greater recognition of the evidential benefit associated with the inclusion of varied demographics within trials.
Recognising inequalities in Africa in particular, CEPI announced last month that it would be providing $6.4m in funding to expand a trial of Bavarian Nordic’s MVA-BN mpox vaccine to include pregnant and breastfeeding mother and under twos in the Democratic Republic of Congo (DRC).
It is part of a broader landscape of increasing trial diversity. Gilead recently became the first sponsor to include pregnant and lactating women in a Phase III human immunodeficiency virus (HIV) study – PURPOSE 1.
However, there is work to be done. In a 2022 report on clinical trial diversity, GlobalData reported that globally, in clinical trials with available race data, White participants account for 59% of trial subjects, more than the combined total for all other racial categories.





