GamaMabs Pharma has dosed the first patient in its Phase ll clinical trial of GM102 and in combination with Trifluridine/Tipiracil (Lonsurf) to treat patients with advanced or metastatic colorectal cancer (CRC).
The European multicentre, two-parallel, non-randomised trial aims to enrol two cohorts of patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Cohort one will include patients who have progressed after at least two lines of prior systemic therapies for metastatic or locally advanced disease and have received all prior available therapies, while the cohort two will include subjects to administer Lonsurf.
The trial will evaluate objective response rates, immunological changes in the tumour microenvironment, progression-free and overall survival rates of the enrolled patients.
It is based on results of collaborative research with various European academic research centres and hospitals.
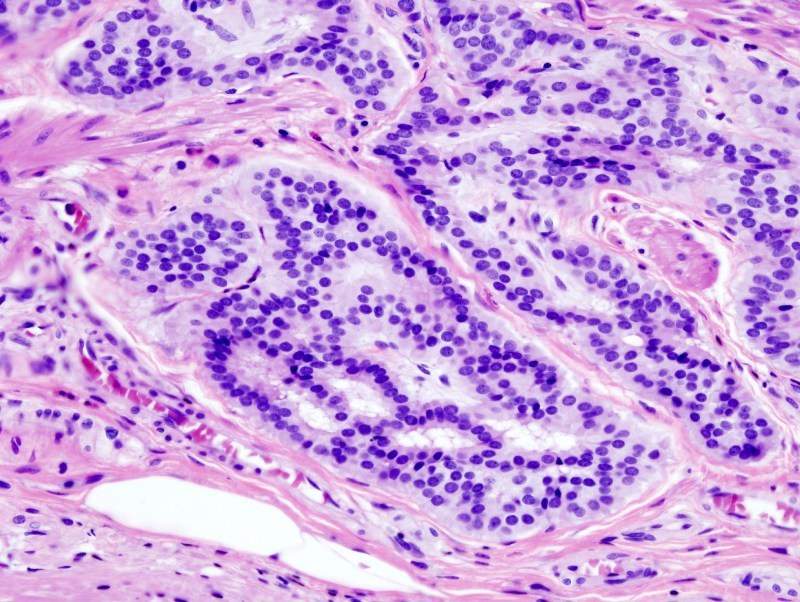
The results showed various Anti-Mullerian hormone receptor type 2 (AMHRII) expression in human CRC tumours and a significant expression of the target on tumour cells at the membrane level.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataGamaMabs Pharma chief medical officer Isabelle Tabah-Fisch said: “The C201 study will investigate the anti-tumour activity of GM102 in this new indication and its potential synergism with Lonsurf for patients who have progressed on prior therapies.
“Most CRC tumours express moderate-to-large macrophage infiltration. GM102 therefore has a potential to enhance macrophage phagocytosis on tumour cells in addition to acting synergistically with Lonsurf.
“This is a key step in the development of our molecule, which has already shown excellent tolerability in patients with gynecological cancers and first signs of activity in the first patients in the phase la/lb C101 study, recently presented at the ASCO 2018 conference.”
GM102 is a glyco-engineered (low-fucose) monoclonal antibody designed to target tumour antigen AMHRII, which is re-expressed in around 70% of CRC patients.





