TIBSOVO® (ivosidenib) is a small molecule inhibitor indicated for the treatment of patients with relapsed or refractory acute myeloid leukaemia (AML) and locally advanced or metastatic cholangiocarcinoma with isocitrate dehydrogenase-1 (IDH1) mutation.
The drug is one of the first specific IDH1 enzyme inhibitors to be approved by the US Food and Drug Administration (FDA). It was developed by Agios Pharmaceuticals and granted orphan drug designation in June 2015, followed by fast track designation in May 2017.
Agios submitted a new drug application (NDA) for ivosidenib to the FDA in December 2017, which was accepted for review in February 2018 and granted a priority review designation. TIBSOVO was initially approved by the FDA in July 2018.
A marketing authorisation application (MAA) was submitted to the European Medicines Agency (EMA) in Q4 2018 but was withdrawn in October 2020 due to insufficient data to conclude a positive benefit-risk balance for AML with IDH1.
In March 2019, TIBSOVO (ivosidenib) in combination with azacitidine received breakthrough therapy designation from the FDA for treating newly diagnosed AML with an IDH1 mutation.
Two months later, the FDA approved a supplemental new drug application (sNDA) to update the label of TIBSOVO to include newly diagnosed AML patients with an IDH1 mutation who are ineligible for standard therapy.
In August 2021, the FDA approved TIBSOVO for the treatment of previously treated adult patients with locally advanced or metastatic cholangiocarcinoma with an IDH1 mutation, after granting priority review status for the company’s sNDA for the indication in May 2021.
Relapsed/Refractory acute myeloid leukaemia causes and symptoms
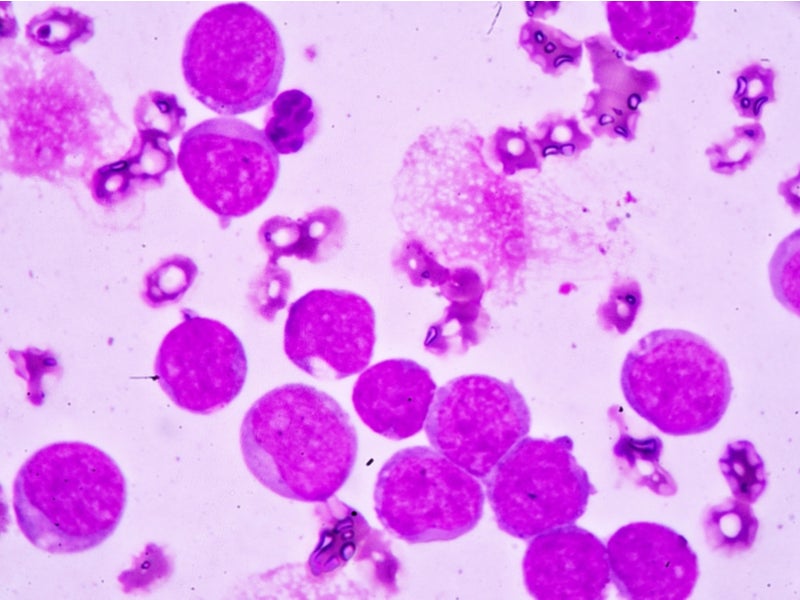
AML is a type of cancer characterised by the uncontrolled production of white blood cells. Called myeloblasts, the abnormal cells build up in the bone marrow and blood, leaving less space for healthy white blood cells, red blood cells and platelets.
The abnormal cells quickly migrate through the blood to other parts of the body causing cancer to spread. Between 6% and 10% of AML patients have a mutation in the IDH1 gene, which produces an enzyme that fosters the uncontrolled growth of leukaemia cells.
The most common symptoms of the disease include weight loss, fatigue, fever, night sweats and loss of appetite.
The National Cancer Institute at the National Institutes of Health (NIH) estimates that approximately 20,000 new patients are diagnosed with AML a year. Most AML patients eventually relapse and have a poor prognosis. The five-year survival rate of prognosis patients is approximately 27%.
Cholangiocarcinoma is a rare and aggressive cancer of the bile ducts that develops within and outside the liver. Approximately 13% of cases of cholangiocarcinoma show IDH1 mutations.
TIBSOVO mechanism of action
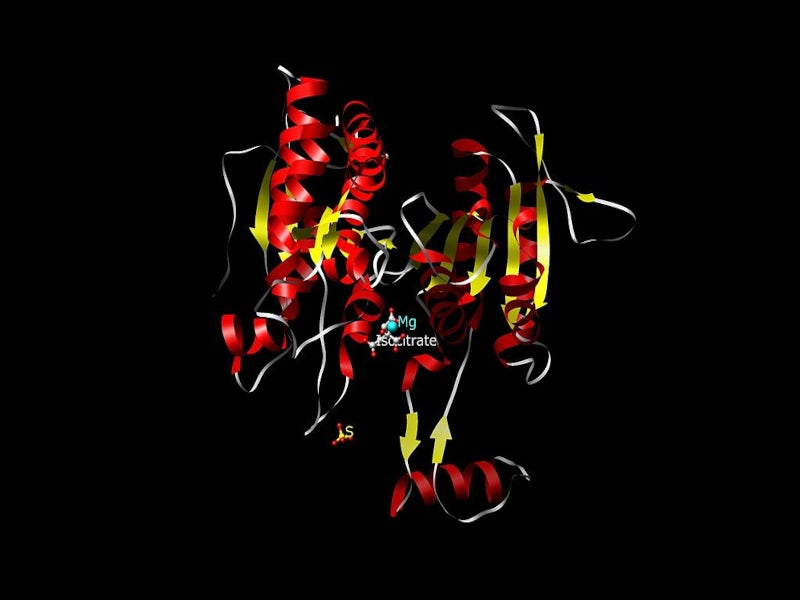
TIBSOVO contains ivosidenib, which is an inhibitor of mutations of the IDH1 enzyme. IDH1 mutations are known to increase the levels of 2-hydroxyglutarate (2-HG) in leukaemia cells, which leads to an increased number of improperly differentiated white blood cells.
R132H and R132C substitutions are the most common types of mutations of the IDH1 enzyme. TIBSOVO inhibits R132 substitutions in the IDH1 enzyme and counters the uncontrolled growth of leukaemia cells.
TIBSOVO is available as a 250mg tablet for oral administration.
Clinical trials on TIBSOVO
The FDA’s approval of TIBSOVO was based on data from an open-label, multi-centre, clinical study named AG120-C-001, which enrolled 174 adult patients with relapsed or refractory AML having a mutated IDH1 gene. The patients were selected based on positive results from the FDA-approved Abbott RealTimeTM IDH1 Assay.
The patients were given a starting dose of 250mg twice daily until progression of the disease or until the development of toxicity advanced beyond permissible limits. The primary endpoint of the study was combined complete remission (CR) and CR with partial hematologic improvement (CRh) rate.
The results of the trial indicated that 32.8% of the patients administered with TIBSOVO experienced either CR or CRh with a median follow-up period of 8.3 months. Further, 37% of the 110 patients that required a blood or platelet transfusion at the beginning of the study became independent of transfusion after 56 days of treatment with TIBSOVO.
The results also indicated that 12% of the 174 patients proceeded with stem cell transplant following TIBSOVO treatment.
The most common adverse reactions during the trials were differentiation syndrome, leucocytosis, arthralgia, diarrhoea, oedema, mucositis, rash, pyrexia, cough and constipation.
The approval of TIBSOVO for cholangiocarcinoma was based on the ClarIDHy clinical study, which was a randomised Phase III trial conducted on previously treated IDH1-mutated cholangiocarcinoma patients.
The patients treated with TIBSOVO showed statistically significant improvement in progression free survival (PFS), which was the primary endpoint of the study, compared to those treated with placebo. The median PFS for TIBSOVO was 2.7 months, while that for placebo was 1.4 months.