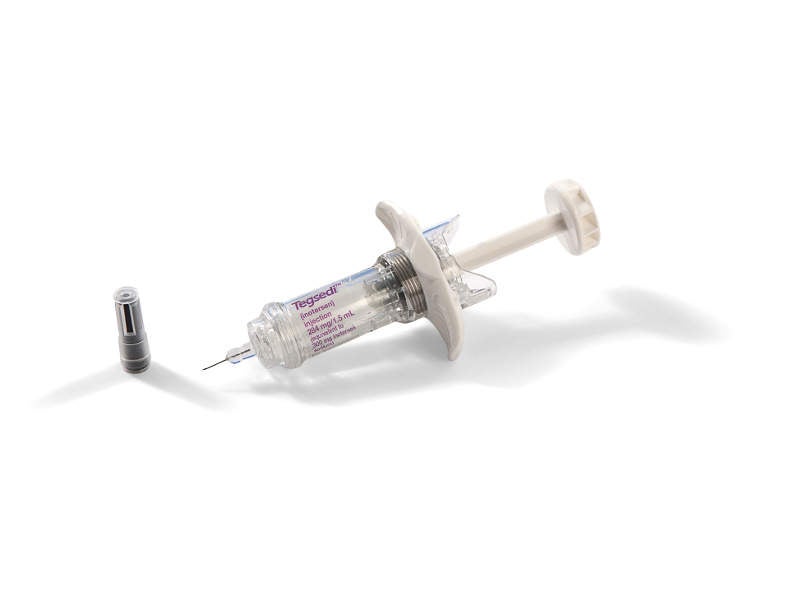
Tegsedi™ (inotersen) is an antisense oligonucleotide indicated for the treatment of polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adult patients.
Discovered and developed by Ionis Pharmaceuticals, the drug received orphan drug designation and fast-track status from the US Food and Drug Administration (FDA).
Ionis Pharmaceuticals also submitted a new drug application (NDA) for the drug in November 2017, which received priority review status from the FDA in January 2018.
Tegsedi™ was approved by the FDA in October 2018 and received marketing authorisation approval from the European Commission (EC) in July 2018. Health Canada approved the drug for the treatment of Stage 1 and Stage 2 polyneuropathy of hATTR amyloidosis in adults in October 2018.
Ionis Pharmaceuticals’ subsidiary Akcea Therapeutics secured the license to commercialise Tegsedi™ globally in March 2018.
Hereditary ATTR causes and symptoms
hATTR is a rare and fatal genetic disease that occurs due to the misfolding or deformation of the transthyretin (TTR) protein, which is formed in the liver. The condition causes accumulation of amyloidal deposits in different parts of the body such as the heart, nerves and gastrointestinal tract.
Some common symptoms of the disease include numbness, pain and weakness in the limbs, cardiovascular issues, diarrhoea, nausea, vomiting, and kidney dysfunction. The progressive disease affects an estimated 50,000 patients worldwide.
Tegsedi’s mechanism of action
Tegsedi™ is a transthyretin-directed antisense oligonucleotide drug designed to reduce the production of TTR by binding to the messenger RNA (mRNA), leading to the reduction of serum TTR and TTR deposits in tissues.
Tegsedi™ is intended for once-weekly subcutaneous administration. It is available as a clear solution of inotersen in a single-dose prefilled syringe. Each vial contains 284mg/1.5ml of solution.
Clinical trials on Tegsedi
The approval of Tegsedi was based on the results of a Phase III clinical study named NEURO-TTR, which enrolled 172 hATTR amyloidosis adult patients with polyneuropathy symptoms. It was a 15-month, randomised, double-blind, placebo-controlled study conducted to study the efficacy and safety profile of Tegsedi™.
Out of 172 patients, 112 patients received Tegsedi™, while the remaining 60 received placebo. The patients received once a week subcutaneous injection for 66 weeks.
The primary endpoints of the study were a change from baseline to week 66 in the modified Neuropathy Impairment Scale+7 (mNIS+7) composite score, and the Norfolk Quality of LifeDiabetic Neuropathy (Norfolk QoL-DN) total score.
The study demonstrated that 90% of the patients treated with inotersen experienced a 50% reduction in TTR, while approximately 50% achieved more than 75% TTR reduction.
In addition, 36.5% of patients treated with Tegsedi™ experienced improvements in mNIS+7 scores compared with 19.2% of placebo-treated patients.
Approximately 50% of patients reported improvements in Norfolk QoL-DN scores, compared with 26.9% placebo-treated patients.
The most common adverse reactions noticed in at least 20% of the patients treated with Tegsedi™ were injection site reactions, nausea, headaches, fatigue, thrombocytopenia and fever. Serious adverse reactions were more frequent in a total of 32% of patients treated with Tegsedi™, compared with 21% patients on placebo.
Another clinical study, NEURO-TTR Open-Label Extension (OLE), also supported the FDA’s approval of the drug. It is being performed to evaluate the long-term efficacy and safety of Tegsedi™ on patients who completed the NEURO-TTR study.