Results from a Phase I clinical trial have shown the investigational mAb114 solution to be safe in the treatment of Ebola.
The trial was launched in May last year at the National Institutes of Health (NIH) Clinical Center in Bethesda, Maryland, US.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
NIH unit National Institute of Allergy and Infectious Diseases (NIAID) Vaccine Research Center (VRC) conducted and sponsored the trial.
NIAID VRC’s single monoclonal antibody mAb114 was also found to be well-tolerated and easy to administer.
The Phase I trial enrolled 18 healthy adults who received a single intravenous infusion of mAb114 for around 30 minutes.
Three of the subjects received a 5mg/kg dose, while five received a 25mg/kg and ten participants received a 50mg/kg dose of mAb114.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAll infusions were reported to be well-tolerated. Four participants experienced mild side effects, including discomfort, muscle or joint pain, headache, nausea, and chills in the three days after the infusion.
The results also demonstrated relatively uniform levels of absorption, distribution, and elimination of mAb114.
NIH in a statement said: “The investigational treatment is currently being offered to Ebola patients in the Democratic Republic of the Congo (DRC) under compassionate use and as part of a Phase II/III clinical trial of multiple investigational treatments.
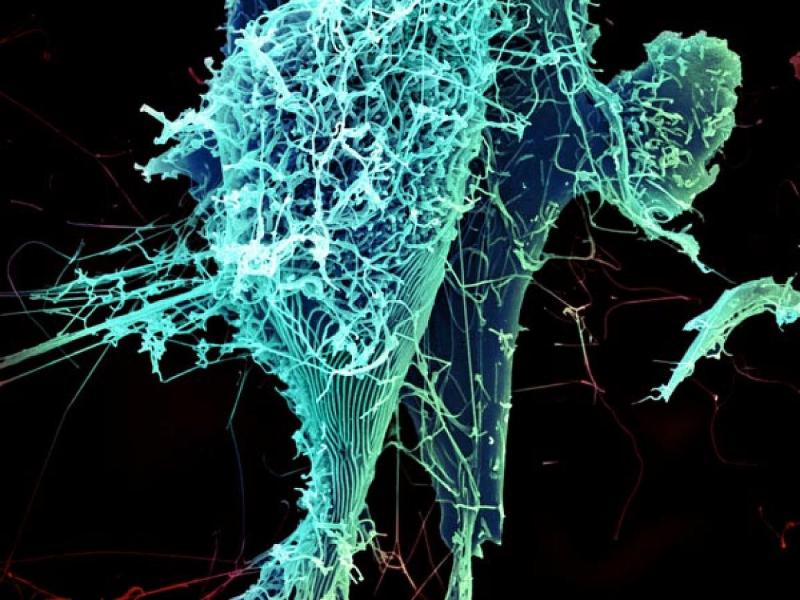
“mAb114, a single monoclonal antibody, binds to the core receptor binding domain of the Zaire ebolavirus surface protein, preventing the virus from infecting human cells.
“Scientists isolated the antibody from a human survivor of the 1995 Ebola outbreak in Kikwit, DRC.”
NIAID VRC is also planning to begin a Phase I trial of mAb114 for the treatment of Ebola in Africa.





