Skyrizi® (risankizumab-rzaa) is a monoclonal antibody (mAb) indicated for the treatment of moderate-to-severe plaque psoriasis, active psoriatic arthritis (PsA) and moderate-to-severe active Crohn’s disease (CD) in adult patients.
The drug is jointly developed and marketed by AbbVie and Boehringer Ingelheim under an agreement signed in March 2016.
AbbVie made an upfront payment of $595m for the drug and holds the global development and commercialisation rights. Boehringer Ingelheim holds co-promotion rights for the drug for the asthma indication and is eligible to receive milestone payments and royalties on sales.
Skyrizi injection is available as a sterile, colourless-to-slightly-yellow, opalescent solution. It is sold in 1ml glass syringes attached to a 29 gauge, 0.5in needle and needle guard.
The Skyrizi 150mg pen was launched in the US as a single-dose injection in August 2021. The pen was designed with broad grip handles and audio cues for support during the administration procedure. An indicator is also provided to inform patients and healthcare providers when the administration is complete.
AbbVie is also conducting clinical trials on Skyrizi for the treatment of ulcerative colitis and announced positive results from Phase III clinical trials for the indication in June 2023.
Regulatory approvals for Skyrizi
In November 2016, Skyrizi received an orphan drug designation from the US Food and Drug Administration (FDA) for the treatment of Crohn’s disease in paediatric patients.
In March 2019, the drug was approved in Japan for the treatment of plaque psoriasis, generalised pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis (PsA) in adult patients. It was also approved in the US, Canada, and Europe in April 2019 for the same indication.
In November 2021, the European Commission (EC) approved Skyrizi for the treatment of adult patients living with active PsA. Under the terms of the EC’s approval, the drug can be used either alone or in combination with methotrexate (MTX) for patients who have a history of inadequate response or intolerance to at least one disease-modifying anti-rheumatic drug (DMARD).
The marketing authorisation for Skyrizi to treat PsA is applicable in all European Union (EU) member states, as well as Iceland, Norway, Liechtenstein, and Northern Ireland. The EC approval came after the EU approved Skyrizi in May 2021.
In November 2021, AbbVie also submitted an application to the European Medicines Agency (EMA) for the approval of Skyrizi to treat moderate-to-severe CD. The EMA approval was sought based on the results of three pivotal Phase III trials, namely ADVANCE, MOTIVATE and FORTIFY.
The US Food and Drug Administration (FDA) approved Skyrizi for the treatment of adults with active PsA in January 2022 and for the treatment of adults with moderate-to-severe active CD in June 2022.
Skyrizi was approved in Canada for the treatment of moderate-to-severe CD in adults in October 2022. The EC approved Skyrizi for the same indication in November 2022.
Plaque psoriasis causes and symptoms
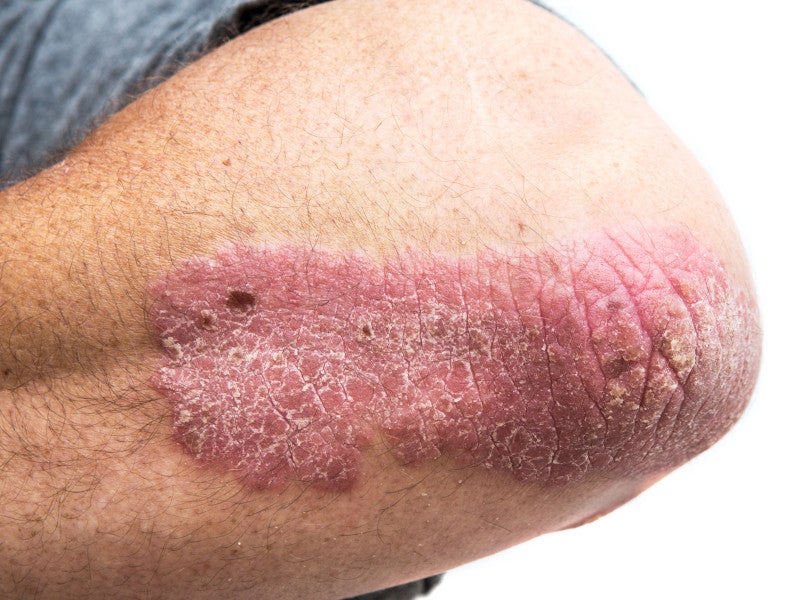
Plaque psoriasis is a heterogeneous, common autoimmune inflammatory disorder that mainly affects the joints and skin. It occurs due to the accelerated development of cells on the skin’s surface.
The disease leads to the formation of dry, itchy and often painful red patches of dead skin cells on the body.
Plaque psoriasis is estimated to affect 125 million people worldwide. Some of the condition’s common symptoms are red, thick lesions with silvery scales, itching, soreness, swollen joints and cracked skin that often bleeds.
Causes and symptoms of Crohn’s disease
CD is a chronic, systemic illness that causes inflammation in the gastrointestinal tract, resulting in recurrent diarrhoea and abdominal discomfort. It is prevalent in more than two million individuals worldwide.
Skyrizi’s mechanism of action
Skyrizi is a humanised immunoglobulin G1 (IgG1) mAb that selectively binds to the interleukin-23 (IL-23) cytokine and blocks its interaction with the IL-23 receptor.
The IL-23 cytokine occurs naturally in the human body and is responsible for inflammatory and immune responses.
Clinical trials of Skyrizi
The initial FDA approval of Skyrizi was based on the positive outcomes of AbbVie’s global Phase III psoriasis programme, which included four multi-centre, randomised, double-blind clinical trials named ULTIMMA-1, ULTIMMA-2, IMMHANCE and IMMVENT.
A total of 2,109 patients with moderate-to-severe plaque psoriasis were enrolled in the studies to evaluate the drug’s efficacy and safety profile compared with placebo. The trials’ co-primary endpoints were the Psoriasis Area and Severity Index (PASI 90), representing a 90% reduction, and a static Physician Global Assessment (sPGA) score of either zero (representing clear) or one (representing almost clear at 16 weeks).
At 52 weeks into the ULTIMMA-1 and ULTIMMA-2 trials, 82% of patients in ULTIMMA-1 and 81% in ULTIMMA-2 treated with Skyrizi achieved PASI 90 scores, and 56% and 60% of patients achieved PASI 100 scores. Patients treated with Skyrizi also achieved 58% and 60% sGPA zero scores in the trials.
The overall analysis of the results highlighted the achievement of a PASI 90 score by 88% of the Skyrizi-treated patients and a PASI 100 score by 80% at week 16. These were maintained one year later.
In the IMMHANCE trial, patients who achieved either sPGA zero or one were randomised again to continue Skyrizi at week 28. At week 52, 87% of the patients maintained the response, while 61% withdrew from the trial.
In the IMMVENT clinical trial, the drug showed results superior to adalimumab. PASI 90 was achieved in approximately 72% of Skyrizi-treated patients compared with 47% of those treated with adalimumab at week 16.
The FDA’s approval of Skyrizi for the treatment of active PsA was based on results from two multi-centre, randomised, double-blind, placebo-controlled Phase III studies, KEEPsAKE-1 and KEEPsAKE-2. The studies were conducted to assess the drug’s efficacy and safety in adults with active PsA.
Skyrizi achieved the primary endpoint of the American College of Rheumatology 20% (ACR20) response at week 24. It showed significant improvements in additional PsA symptoms, including tender, swollen and painful joints, compared with placebo.
The most common adverse events reported by patients in the plaque psoriasis and psoriatic arthritis trials were upper respiratory infections, headache, fatigue, injection site reactions and tinea infections.
Skyrizi’s approval for moderate-to-severe active CD was based on the safety and efficacy data from two induction clinical trials and one maintenance trial, ADVANCE, MOTIVATE and FORTIFY. The drug showed significant improvements in endoscopic response and clinical remission compared with placebo in the studies.
Clinical trials on Skyrizi for ulcerative colitis indication
AbbVie announced positive results from the INSPIRE Phase III induction study in patients with moderate to severe ulcerative colitis in March 2023. In the double-blind, placebo-controlled study, 20.3% of the participants administered risankizumab achieved clinical remission at week 12, compared to 6.2% of participants receiving placebo.
36.5% of participants treated with risankizumab achieved endoscopic improvement compared to 12.1% of participants receiving placebo, and 25% of patients gained a histologic-endoscopic mucosal improvement compared to 7.7% of those receiving placebo.
In June 2023, AbbVie announced positive results from the Phase III COMMAND maintenance study conducted in patients from the INSPIRE study who responded to the induction treatment. The study met the primary endpoint of clinical remission at week 52, along with secondary endpoints.
40% of patients administered with 180mg risankizumab and 38% of patients administered with 360mg risankizumab achieved clinical remission, compared to 25% of patients in the induction-only control group. 51% of the patients administered with 180mg risankizumab and 48% administered with 360mg risankizumab achieved endoscopic improvement compared to 32% of patients in the induction-only group.