Esperoct® (turoctocog alfa pegol) is a recombinant factor indicated for the treatment of haemophilia A in adults and children.
Developed by Novo Nordisk, a biologics license application was submitted to the US Food and Drug Administration (FDA) for the drug in February 2018. It was approved by the FDA in February 2019.
An application was also sent to the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP), which provided a positive opinion for the marketing of the drug in Europe in April 2019.
The drug holds orphan drug designation for haemophilia A treatment from both the EMA and the FDA. It is available as a lyophilised powder in single-dose vials of 500, 1,000, 1,500, 2,000 and 3,000 IU dosages for intravenous administration.
Market launch of the drug in the US is not expected before 2020 due to third-party intellectual property (IP) agreements.
Haemophilia A causes and symptoms
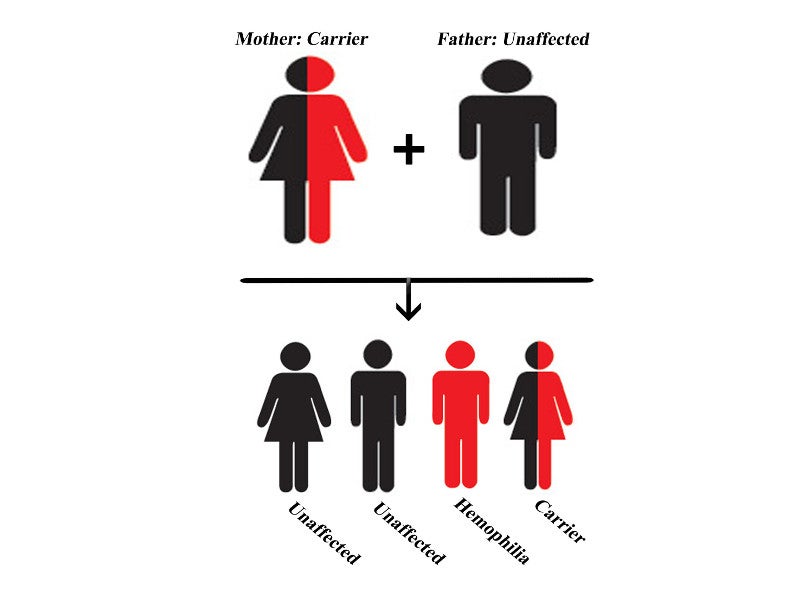
Haemophilia A is a genetic bleeding disorder inherited as an X-linked recessive trait. It occurs when a defective gene located on the X-chromosome fails to produce factor VIII, which is an essential blood-clotting protein.
The disease is more common in males due to the presence of a single X-chromosome. Since females carry two (XX), a deficiency in the faulty information is balanced by the other normal chromosome. The disease occurs in one out of 5,000 male births.
Common symptoms of the disease are bleeding episodes with or without injury, muscle or joint haemorrhages, prolonged headache, fatigue and double vision.
Esperoct’s mechanism of action
Esperoct is a recombinant form of DNA-derived coagulation factor VIII concentrate with extended half-life. It replaces the missing factor VIII in haemophilia A patients for effective haemostasis.
In Esperoct, factor VIII is conjugated with 40kDa polyethylene glycol molecule to prolong the half-life by 1.6 times in adults and 1.9 times in children, compared to the conventional half-life factor VIII products. It decreases clearance compared to the non-pegylated molecule.
Clinical trials on Esperoct
The FDA’s approval of Esperoct was based on the positive results of a pre-registration clinical programme named Pathfinder. The programme included five prospective, multi-centre, open-label clinical trials, which enrolled 270 previously treated haemophilia A patients.
The drug demonstrated efficacy in routine prophylaxis in patients through a fixed-dose regimen of single injection every four days in adults and young adults or twice a week in children.
Esperoct maintained a low median annualised bleeding rate of 1.18 in adults and young adults when administered at 50IU/kg every four days. The drug was also found effective in the treatment and management of bleeding episodes.
It was well tolerated by all the patients and the safety profile was found similar to that of long-acting factor VIII drugs.
The most common adverse events reported in the patients during the clinical trials were rash, redness, itching, and injection site reactions.
Marketing commentary on Novo Nordisk
Based in Denmark, Novo Nordisk is a pharmaceutical company focused on the development of medications for diabetes and other serious chronic diseases including haemophilia, and obesity.
The company commercialises its products in more than 170 countries and has more than 43,200 employees across 80 countries.