Doripenem is part of Johnson &’ Johnson’s anti-infective R&D portfolio following the acquisition of Peninsula Pharmaceuticals in 2005. The drug, a member of the carbapenem class of Β-lactam antibiotics, is indicated for the treatment of serious bacterial infections in hospitalised patients.
Marketed under the brand name Doribax in Europe and the US, it is an approved treatment for complicated intra-abdominal (cIAI) and complicated urinary tract infections (cUTI), including pyelonephritis in adults.
In Europe it is also licensed for treatment of nosocomial pneumonia (NP), including ventilator-associated pneumonia (VAP); final approval for these indications was obtained in 2008. In the US, however, the FDA has requested additional information before it will sanction approval of Doribax for these additional indications.
In Japan, Shionogi, the drug’s originator, markets doripenem under the brand name Finibax. Peninsula Pharmaceuticals acquired development and marketing rights to doripenem in the US in a licensing agreement signed with Shionogi in 2003. In the US, Doribax is marketed by Ortho-McNeil-Janssen Pharmaceuticals.
In October 2009, Doribax was approved in Canada for the treatment of cIAI, cUTI, NP and VAP. In February 2010, the drug received the A-1 rating for the treatment of certain high-risk cIAIs under new diagnosis and management recommendations. These guidelines help clinicians in treating patients who are at the risk of life-threatening bacterial infections.
Broad-spectrum antibacterial activity
First introduced into clinical practice about 20 years ago, carbapenems are the most recent addition to the Β-lactam group of antibiotics, which also includes penicillins and cephalosporins.
Carbapenems share the broad-spectrum activity of the Β-lactam group but differ from other members in possessing stability to most clinically important bacterial Β-lactamases. This makes them useful in first-line treatment of serious bacterial infections and as reserved therapy for defined resistant pathogens.
Doripenem, a 1-Β-methyl carbapenem, has a number of features that suggest it could be a useful addition to the current range of carbapenems:
- enhanced activity against non-fermentative gram-negative bacilli
- bactericidal activity against most pathogens
- stability to human renal dehydropeptidases
- stability to common bacterial Β-lactamases, including extended-spectrum Β-lactamases
- post-antibiotic effects against Pseudomonas aeruginosa
- potent activity against penicillin-resistant streptococci
- effectiveness at low doses.
Pivotal Phase III trials
Doripenem entered Phase III development in the US based on results from successfully completed Phase II trials conducted by Peninsula Pharmaceuticals.
Regulatory approval was secured on the basis of four Phase III trials that involved patients with cIAIs and cUTIs. Across the four trials, doripenem emerged superior to meropenem and intravenous levofloxacin in both infections. It was also well tolerated.
In pseudomonal eradication, doripenem was proved superior compared to meropenem. In two cIAI clinical studies conducted, doripenem cured 95% and 76% of patients respectively, while meropenem cured only 79% and 69%.
Intravenous doripenem is also being evaluated in NP, which is among the most common infections in intensive care units, accounting for about 15% of all hospital-acquired infections. Mortality exceeds 30% and is highest among mechanically ventilated patients. Selecting the right antibiotic is an important determinant of clinical outcome in such cases.
In the Japanese Phase III trials, which formed the basis of regulatory submissions to the Japanese Ministry of Health, Labor and Welfare, intravenous doripenem was evaluated in pneumonia, chronic respiratory tract infections, and UTIs.
Doripenem expands Johnson & Johnson’s anti-infective portfolio
Johnson & Johnson added doripenem to its range of marketed antibiotics along with ofloxacn (Floxin) and levofloxacin (Levaquin).
Although the hospital antibacterial market represents only a third of all antibacterial sales, increasing antibiotic resistance in the hospital environment has increased the demand for more effective antibiotics, making it an attractive sector for new antibacterial agents.
However, Johnson & Johnson’s other antibiotic, ceftobiprole, has been rejected by US and EU regulators, after it emerged that its Phase III studies were not carried out in compliance with good clinical practice in certain sites.
Marketing commentary
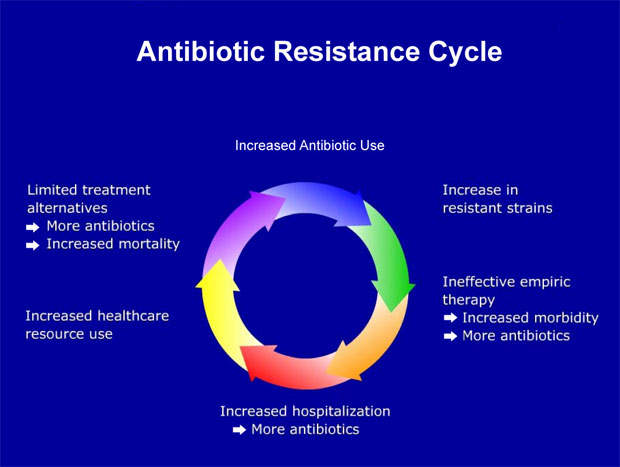
The past decade has seen a dramatic rise in antibiotic resistance in the hospital setting, compromising treatment outcome in critically ill patients.
Of all the Β-lactam antibiotics, the carbapenems are considered to have the broadest spectrum of activity that includes coverage of non-fermentative gram-negative bacilli such as Acinetobacter sp and Pseudomonas aeruginosa. This makes them attractive agents for empirical therapy in the hospital setting against difficult-to-treat pathogens.
At a time of rising rates of bacterial resistance to commonly used antibiotics, new agents are urgently needed to treat bacterial infections effectively and halt the spread of resistant strains. This need is arguably the greatest in the hospital environment, especially in intensive care units where the rates of antibiotic resistance are highest.