Novavax’s trivalent seasonal influenza virus-like particle (VLP) vaccine was developed to address the need to produce vaccines in a short time. The technology allows for seasonal influenza strain vaccine to be produced in ten to 12 weeks, rather than the 20–24 weeks needed to use traditional egg-based vaccine production methods.
In June 2009, Novavax developed a VLP vaccine to fight the 2009 H1N1 virus after receiving genetic material from the US Centers for Disease Control and Prevention (CDC). In about three weeks, Novavax developed prototype VLPs of the H1N1 virus strain. An important part of the development was the creation of reagents needed to measure the strength of the vaccine. The VLP received US patent issuance on 27 July 2010.
Seasonal influenza
Seasonal influenza has caused major pandemics over the years. There are three varieties of influenza viruses: A, B and C. Human influenza A viruses lead to seasonal epidemics during the winter months in the US. Type B viruses are less common than type A. Type C influenza can lead to a minor respiratory illness and are not known to cause epidemics.
There are three prominent seasonal influenza A subtypes – H1N1, H2N2, and H3N2 – which have been responsible for these pandemics. Seasonal influenza is estimated to affect about 5–20% of the global population and deaths related to seasonal flu are estimated between 250,000 and 500,000 a year.
In the US alone, according to the CDC, seasonal influenza infects about 15–60 million people every year and results in around 36,000 deaths.
Antigenic drift, antigenic shift, or combination of two or more strains of viruses results in the creation of a new strain of the influenza virus. Antigenic drift can create a strain that partially avoids immunity developed by earlier strains. To track such changes vaccine compositions need to be updated regularly on an annual basis. Antigenic shift, however, can create a strain with no pre-existing immunity which often leads to a pandemic. It also increases the mortality rate in young and healthy adults due to lack of preparation to deal with the new strain.
The most recent example of a pandemic created by seasonal influenza was the 2009 influenza A (H1N1). First discovered in April 2009 in Mexico, the virus quickly spread to other countries. Outbreaks such as this have highlighted the need to develop new vaccines rapidly in the early stages of a pandemic. Effective vaccines need to be produced quickly to curb the spread of the virus and safeguard public health.
Recombinant trivalent seasonal influenza VLP vaccine
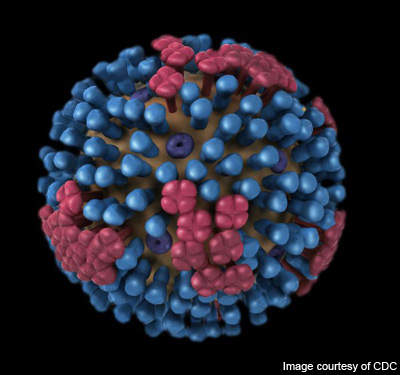
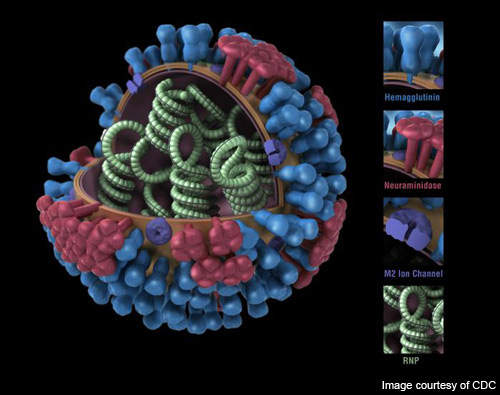
Novavax’s VLP vaccine carries three VLPs each of the hemagglutinin (HA), neuraminidase (NA) and matrix 1 (M1) proteins from the respective strains aggregated together in a single formulation. The VLP vaccine replicates the external structure of the viruses but does not contain any live genetic substance which can cause viral replication and infection.
When the vaccine is administered to a patient, the immune system responds as if it were attacking a real virus and creates antibodies to fight the virus. As a result, when the real strain attacks, the patient develops immunity.
VLP-based vaccines can be produced much faster than egg-based vaccines as they are produced using proprietary, portable, recombinant baculovirus vector and insect cell-culture technology. The technology does not require live flu virus seed which needs to grown in eggs in a typical egg-based vaccine. VLP-based vaccines are also safer as they do not bear the risk of becoming virulent again.
Clinical trials
In April 2009, Novavax announced positive preclinical results with the VLP vaccine against the H3N2, H1N1 and B influenza strains. The study was carried out by scientists from the University of Pittsburgh, Center for Vaccine Research and Novavax.
In the study, mice and ferrets were injected with VLP vaccine. HAI antibodies were generated against all three influenza strains present in the vaccine and against a range of drifted strains during the study in the mice and ferrets.
In May 2009, Novavax announced that it started its second Phase II clinical study with adults aged between 18 and 49. The second Phase II clinical trial was carried out to test the safety and efficacy of the VLP vaccine against H3N2, H1N1 and B influenza strains, which surfaced in the 2008–2009 influenza season. The first Phase II trial was completed in December 2008 by Novavax. The study tested the safety and efficacy of the vaccine against seasonal influenza strains that emerged during the 2005–2006 influenza season.
The results of this trial, published in April 2010, confirmed that the VLP vaccine induced a high haemagglutination inhibition antibody response against the H1N1, H3N2 and B strains.
In November 2009, Novavax reported positive results from its second Phase II study. The study enrolled 241 patients and showed that the VLP vaccine developed immunity in the patients.
In the same month, the company also announced the launch of a Phase IIa study of the VLP vaccine in patients aged 60 and above. The study compared the safety and efficacy of two separate doses of the VLP vaccine with that of other trivalent inactivated vaccines present in the market.
The final results of the Phase II study were announced in March 2010. It enrolled 480 patients and tested the tolerability, safety and immunogenicity of trivalent influenza VLP. The vaccine was administered in a single 15µg dose or 60#181;g dose and was compared to a dose of a commercially available inactivated trivalent influenza vaccine.
In the primary immunogenicity study, it was found that the vaccine aroused hemagglutination inhibition antibody response after 21 days of immunisation. After administering low and high doses, the vaccine did not show any substantial increase in local severe and adverse events.
Marketing commentary
With new strains of influenza virus emerging every year, it is essential to develop faster methods of vaccine creation. Egg-based vaccines are no longer effective in terms of cost and efficiency. Egg-based vaccine technology, for example, requires about 900 million eggs to produce vaccines for the US population against an influenza outbreak. In case of a deadly virus strain, the process is even more ineffective and time consuming.
The Advisory Committee on Immunization Practices (ACIP) suggests that seasonal influenza vaccination should be administered to children from six months to 18 years of age, pregnant women, people over 50 years of age and any individuals who have chronic health conditions or are prone to influenza disease. Global demand for seasonal influenza vaccines is estimated to increase the cost of administering vaccines from $2.8bn in 2007 to $6.5bn by 2013.
Novavax’s VLP technology will help in addressing this growing need for new vaccines. The technology can produce significant quantities of vaccine using only 1,000–2,000l disposable bioreactors, which further reduces time and the cost of building and validating a new production facility.