Intermezzo is a formulation of zolpidem that is indicated for the treatment of insomnia or sleeping disorders. The drug was discovered by Transcept Pharmaceuticals in 2008.
Transcept submitted a new drug application to the US Food and Drug Administration (FDA) in September 2008. In October 2009, the FDA issued a complete response letter, asking Transcept to furnish more details .
On 18 January 2011, the company submitted the revised new drug application to the FDA seeking approval of Intermezzo. If it is accepted, the drug will be subjected to a six-month Class 2 review.
Transcept completed the Phase III clinical trials on the drug in 2009.
Insomnia
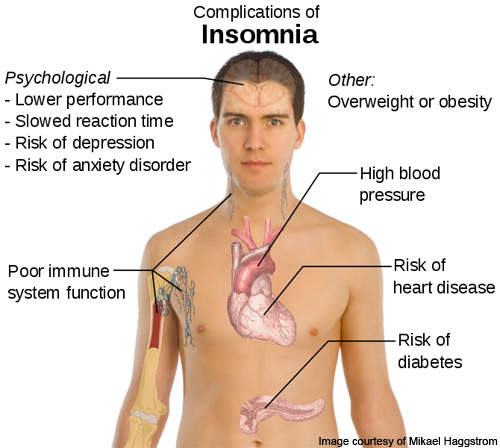
Insomnia is a disorder characterised by regular difficulty in getting sleep or returning to sleep after waking up in the middle of the night. It is a condition in which the patient gets very poor quality sleep. The disease is very commonly found in older adults and women.
According to a study by Stanford Sleep Epidemiology Research Center, middle of the night awakening is a very commonly reported insomnia symptom, and almost 35% of the adults in the US experience this as frequently as almost thrice a week.
In 2007, the US Department of Health estimated that there were at least 64m people in the US who were affected by insomnia.
There are a lot of factors which can cause this disease, such as mental health conditions, lifestyle factors and other underlying causes. The symptoms and complications of insomnia, however, may vary from one individual to the other.
The disease can affect the quality of life of the affected person, as it may result in irritation, lack of concentration, fatigue and also affect the ability and productivity of the patient.
Targeting insomnia
Intermezzo is used for treating the middle of the night awakening. The drug is produced by using Transcept’s Bimucoral technology and contains a sublingual lozenge formulation with mint flavouring. The drug is taken orally and gets dissolved in two minutes. It is available in 1.75mg and 3.5mg doses.
Intermezzo contains a low dose of zolpidem, providing insomnia patients with a fast acting solution. The drug can be taken only when it is necessary, either for getting undisturbed sleep or in the case of midnight awakening.
The drug is very safe and effective as long as the patient remains in bed for at least four hours after taking the drug. The drug provides rapid return to sleep without the risk of any sedation effect in the next morning.
Intermezzo has accomplished higher bio-availability during the first 10 to 20 minutes after administration when compared to Ambien 10mg, which is a drug used for short-term treatment of insomnia. Intermezzo contains just one third dose of zolpidem in comparison to Ambien.
Clinical trials
The two-phased Phase III clinical trials started in April 2006 and were completed in September 2009. The first Phase III trial was a double-blind, placebo-controlled, cross over sleep laboratory study with 82 insomnia patients.
The study revealed that when 3.5mg and 1.75mg doses of Intermezzo were used, the result was significant in comparison with placebo as the night awakening was shortened. There was no sedative effect the next morning.
When the 3.5mg dose was used it resulted in significant improvement in sleep efficiency, sleep time and sleep quality.
The second Phase III trial was a double-blind and placebo-controlled outpatient study, which enrolled 294 patients.
Both placebo and Intermezzo 3.5mg were used when the patients woke up during the night and found trouble in getting back to sleep. The results showed that there was no next day sedative effect when using Intermezzo. The placebo was found to be ineffective.
In October 2009, the FDA expressed concern over dosing errors by patients during the middle of the night, which might affect them on the following morning.
The FDA was especially concerned about how the drug would affect patients’ driving the next day.
This prompted Transcept to conduct a highway driving study before re-submitting its new drug application. The study involved 40 healthy volunteers and evaluated the safety of Intermezzo with regard to its potential impact on the volunteer’s ability to drive the next day. The preliminary results, announced in October 2010 indicate that Intermezzo does not increase the risk of driving impairment.
Marketing commentary
If the drug gets nod from the FDA, it could become the first prescription specifically for middle of the night awakening.
In August 2009, Transcept reached an agreement with Purdue Pharmaceuticals allowing the latter to sell and market the drug in the US. Transcept, however, retained the rights to sell or market the drug in other parts of the world.