Synribo (Omacetaxine Mepesuccinate) is a subcutaneously injected drug developed by Teva Oncology, a division of Teva Pharmaceutical Industries, based in the US. The drug is indicated for the treatment of patients with chronic myelogenous leukaemia (CML) in both chronic and accelerated phases.
In October 2012, Synribo was approved by the US Food and Drug Administration (FDA) for treating patients in the chronic and accelerated phases of CML who were previously treated with two or more tyrosine kinase inhibitors (TKIs) and failed in the treatment.
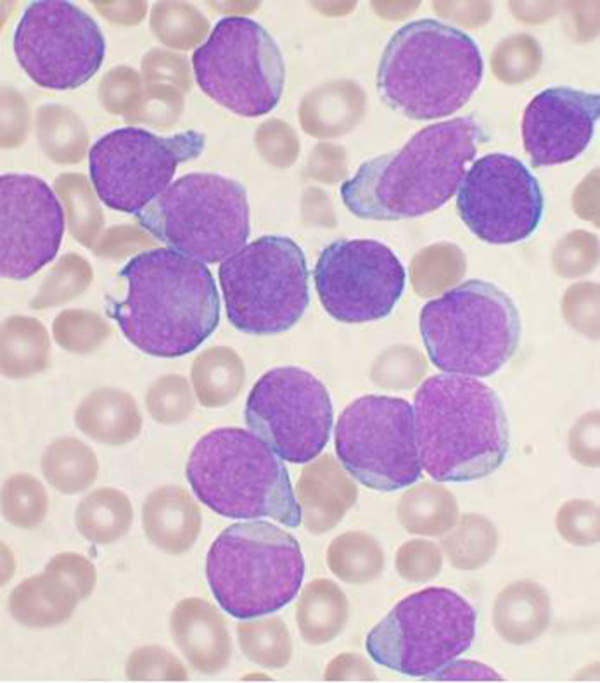
Chronic myelogenous leukaemia (CML) disease
Chronic myeloid leukaemia (CML) is a type of white blood cell cancer that emanates from bone marrow.
The disease is characterised by an uncontrollable increase of immature white blood cells. It is associated with the Philadelphia chromosome. The disease occurs mostly in middle-aged and elderly people.
According to American Cancer Society estimates, about 5,430 new cases of the disease will be diagnosed in 2012, of which 610 are expected to be fatal.
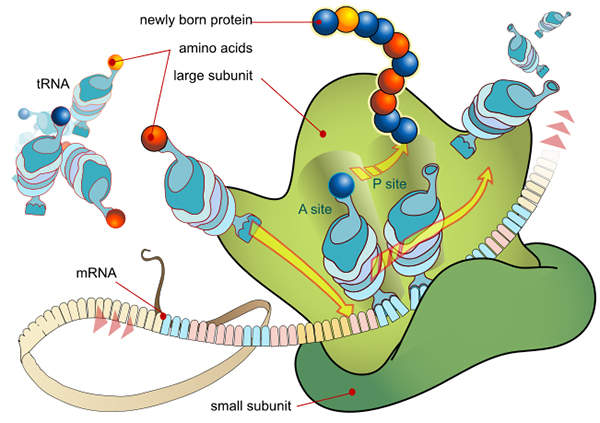
Synribo’s mechanism of action
Synribo is a protein translation inhibitor. The precise mechanism of action of the drug is not fully known, but it is presumed that the drug works by obstructing proteins that promote the development of cancerous cells. Synribo is administered subcutaneously twice daily at a dose of 1.25mg/m2.
Omacetaxine mepesuccinate clinical trials
Teva conducted three initial clinical trials on 163 patients to evaluate the safety and efficacy of Synribo.
The FDA approved Synribo under the accelerated approval programme based on the preliminary data from two Phase II clinical trials, conducted between 2006 and May 2012. They were open label and multicentre studies, which enrolled more than 203 patients. The studies enrolled both chronic and accelerated phase CML patients, who were administered with two or more approved TKIs at a minimum.
The major cytogenetic response (MCyR) and a major hematologic response (MaHR) were calculated using the Kaplan-Meier estimate. The patients were administered with Synribo 1.25mg/m2 subcutaneous injection twice a day for 14 consecutive days of a 28-day cycle.
The preliminary results announced in May 2012 demonstrated that Synribo achieved 18% MCyR in chronic phase of CML patients. The drug also achieved a mean time to MCyR onset of 3.5 months. The median duration of MCyR was 12.5 months.
The results also showed that Synribo achieved more than 14% MaHR in the accelerated phase CML patients. The mean time to MaHR response of onset was 2.3 months, and median duration of MaHR was 4.7 months.
The most common adverse reactions reported during the clinical trials included injection site reaction, anaemia, neutropenia and thrombocytopenia. The reactions also included diarrhoea, nausea, fatigue, asthenia, infection, lymphopenia and pyrexia.
Marketing Teva Pharmaceutical Industries’ Synribo
Teva Pharmaceutical Industries is one of the largest pharmaceutical companies in the world with its headquarters in Israel. It is engaged in manufacturing and developing high-quality healthcare and affordable generic drugs. It is also engaged in developing innovative and specialty pharmaceuticals and active pharmaceutical ingredients.
The company has a presence in more than 60 countries across the world with a product portfolio of more than 1,300 molecules. It focuses on developing branded business in the areas of oncology, CNS, respiratory and pain, as well as biologics. The company achieved net revenue of about $18.3bn in 2011.
Teva Oncology is a US division of Teva Pharmaceutical Industries. Its US headquarters is situated in Frazer in Pennsylvania. Teva holds the US marketing rights of Synribo. It may face tough competition from other approved medications for the treatment of various forms of CML, including Novartis’ imatinib and nilotinib, Bristol-Myers Squibb’s dasatinib and Pfizer’s bosutinib.
Related content
Iclusig (ponatinib) for the Treatment of Chronic Myeloid Leukaemia
Iclusig (ponatinib) is indicated for the treatment of chronic myeloid leukaemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukaemia (Ph+ALL).
Vosaroxin – Treatment for Myeloid Leukaemia, United States of America
Vosaroxin is a first-in-class anticancer quinolone derivative (AQD) under development by Sunesis Pharmaceuticals for the treatment of acute myeloid leukaemia (AML).