Jetrea (ocriplasmin) is a pharmacological agent developed by ThromboGenics, for the treatment of symptomatic vitreomacular adhesion (VMA). Jetrea was approved by the US Food and Drug Administration (FDA) for treating symptomatic VMA in October 2012.
The drug is currently being reviewed for marketing authorisation in Europe.
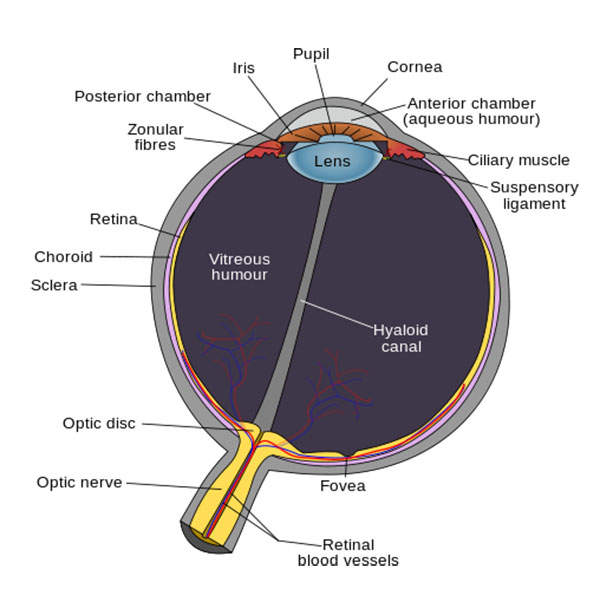
About symptomatic vitreomacular adhesion
Symptomatic VMA is a condition in which the vitreous humour in the eye sticks to the retina. The condition leads to retinal distortion and can cause visual impairment. It can also lead to several eye disorders such as macular hole, macular pucker, macular oedema, and age-related macular generation (AMD).
According to surveys conducted by ThromboGenics, the condition affects about 500,000 patients in the US.
Jetrea’s mechanism of action against VMA
The plasmin enzyme in Jetrea breaks up blood clots in the eye. The drug works by breaking down the vitreoretinal interface. It prevents the build up of abnormal proteins in cells such as fibronectin, laminin and collagen.
The drug also dissolves the protein matrix that is responsible for VMA.
Clinical trials on Jetrea (ocriplasmin)
ThromboGenics initiated Phase II clinical trials on Jetrea in October 2011. The randomised, sham-controlled, multi centre and double-masked clinical study enrolled 220 patients with symptomatic VMT including macular hole. The primary outcome measure of the study includes establishing the proportion of successfully treated patients at day 28. The final results of the study are expected by April 2015.
The FDA approval for Jetrea was based on the data received from two Phase III clinical trials which were conducted in the US and Europe. The studies together enrolled about 652 symptomatic VMA patients. The efficacy and tolerability of the drug was evaluated in these studies.
The first Phase III trial on Jetrea was conducted between December 2008 and April 2010. It was a randomised, placebo controlled, double-masked, multicentre clinical trial. It enrolled 326 patients. The primary outcome measure of the study was to find the resolution of the VMA at day 28. The secondary outcome measure included finding the total posterior vitreous detachment (PVD) at day 28.
The second Phase III clinical trial on Jetrea was initiated from December 2008 to July 2010. It was also a randomised, placebo controlled, double-masked and multicentre clinical trial. The study also enrolled 326 patients. Its primary outcome measure was finding the proportion of patients with nonsurgical resolution at day 28. The secondary outcome measure included finding the total PVD at day 28.
Jetrea met the respective primary endpoints in both the studies. The results of the studies demonstrated that Jetrea provided superior treatment of symptomatic VMA when compared to placebo. The drug was also well-tolerated and the adverse events were mild in severity.
Marketing Jetrea in the US and Europe
Related project
Lucentis (ranibizumab) for the Treatment of Diabetic Macular Edema (DME), United States of America
Lucentis (ranibizumab) is a monoclonal antibody indicated for the treatment of diabetic macular edema (DME). The drug is developed by Genentech, a subsidiary of Roche Group.
ThromboGenics is a biopharmaceutical company headquartered in Leuven, Belgium. The commercial operations of the company are carried out from New Jersey, US. The company is primarily engaged in the development, manufacturing, and marketing of inventive ophthalmic medicines.
Jetrea is first medicine approved for the treatment of symptomatic VMA. ThromboGenics holds the commercial rights of Jetrea in the US. In March 2012, the company entered into a partnership agreement with Alcon, a subsidiary of Novartis, for the commercialisation of Jetrea outside the US. ThromboGenics will receive about €375m ($488.7m) towards milestone payments, as per the agreement.
In November 2012, Fujifim Diosynth Biotechnologies was awarded the contract to manufacture and supply Jetrea in the UK. Alcon holds the marketing rights for the drug in Europe.