Invokana (canagliflozin) is indicated to improve glycemic control in adults with type 2 diabetes, along with diet and exercise. It was first developed by Mitsubishi Tanabe Pharma Corporation in Japan and later licensed to Janssen Pharmaceuticals.
In March 2013, Invokana received approval from the US Food and Drug Administration (FDA) for the treatment of adults with type 2 diabetes.
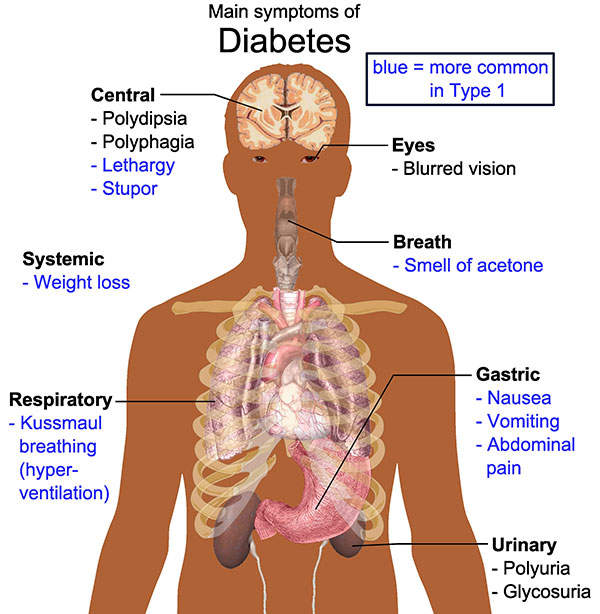
Type 2 diabetes symptoms
Type 2 diabetes mellitus is a metabolic disorder in which the body does not produce enough insulin and / or the cells of the body do not respond adequately to it. It is characterised by high levels of sugar in the blood. Some of the symptoms of the disease include frequent urination, constant appetite and excess thirst.
According to the US Centers for Disease Control and Prevention, about 26m people in the US are affected by diabetes. It is also found that about 60% of type 2 diabetes patients are obese and about 30% are overweight. Obesity can make body cells resist the action of insulin.
Invokana mechanism of action
Invokana is the first in a new class of drugs, called sodium glucose co-transporter 2 (SGLT2) inhibitors, to be approved in the United States. SGLT2 reabsorbs glucose filtered from the blood into the kidneys. By inhibiting SGLT2, Invokana acts as a ‘glucuretic’, promoting the emission of glucose in the urine and reducing blood glucose levels.
The drug is available in 100mg and 300mg dose tablets for oral administration.
Clinical trials of the new diabetes drug
Janssen conducted a Phase I clinical trial on Invokana between December 2011 and August 2012. It was a randomised, double-blind, placebo-controlled and parallel assignment that enrolled more than 35 patients with type 2 diabetes. The study evaluated the pharmacokinetics of Invokana. The primary outcome measure of the study was finding the change in plasma volume, finding the number of patients who experienced at least one occurrence of a treatment-related adverse event and finding the hypoglycemic events reported during the study. The secondary outcome measures included finding the change in plasma volume, change in body weight and change in 24-hour urine volume.
A Phase II clinical trial of Invokana was carried out from July 2011 to April 2012. It was a multicentre, randomised, double-blind, and parallel assignment. The study enrolled 279 patients with type 2 diabetes. The primary outcome measure of the study was finding the change in hemoglobin A1c from baseline at week 18. The secondary outcome measures included finding the change in fasting plasma glucose from baseline at week 18 and change in body mass index (BMI).
Janssen conducted a Phase III clinical study on Invokana between May 2010 and May 2012. It was a randomised, double-blind, placebo-controlled and parallel assignment. The primary outcome measure of the study included the assessment of the effect of Invokana relative to placebo on hemoglobin A1c (HbA1c). The secondary outcome measures included evaluation of the effect of postprandial plasma glucose concentrations, and its effect on the proportion of patients achieving HbA1c.
Janssen filed a new drug application with the FDA for Invokana based on nine global, randomised and double-blind Phase III clinical studies on Invokana. The Phase III programme enrolled 10,285 patients with type 2 diabetes. The clinical studies assessed the efficacy and safety of Invokana 100mg and 300mg doses as monotherapy and in combination with oral antihyperglycemic agents, and insulin.
The results of the Phase III clinical trials demonstrated improved glycemic control as well as reductions in body weight and systolic blood pressure in patients administered with 100mg and the 300mg doses of Invokana.
The clinical studies included CANTATA (CANagliflozin Treatment And Trial Analysis) and CANVAS (CANagliflozin cardioVascular Assessment Study). The two Phase III studies compared Invokana 300mg dose with the existing standard treatments, sitagliptin and glimepiride. The patients administered with Invokana 300mg dose achieved superior reductions in A1c levels and body weight than the standard treatments.
The common adverse events found during the study in Invokana-administered patients included genital mycotic infections, urinary tract infections and increased urination.
The FDA has asked Janssen to carry out five post-marketing studies on Invokana.
Marketing Ivokana in the US and worldwide
Related project
Nesina (alogliptin) for Treatment of Type 2 Diabetes Mellitus, United States of America
Nesina (alogliptin) is a dipeptidyl peptidase-4 (DPP-4) inhibitor developed and marketed by Takeda Pharmaceuticals for the treatment of diabetes 2 mellitus.
Mitsubishi Tanabe Pharma Corporation has licensed the rights to develop and market Invokana to Janssen in North and South Americas, Europe, Middle East, Africa, Australia, New Zealand and Asia, except in Japan. Mitsubishi Tanabe Pharma holds the marketing rights for Invokana in Japan.
The other medications available for the treatment of similar indication include Nesina, marketed by Takeda Pharmaceuticals and Furiex Pharmaceuticals, Forxiga, developed by AstraZeneca and Bristol-Myers Squibb and Tradjenta, developed by Boehringer Ingelheim Pharmaceuticals and Eli Lilly.