Breo Ellipta (fluticasone furoate and vilanterol inhalation powder) is a dry powder inhaler indicated for the long-term maintenance treatment of airflow obstruction and reduction of exacerbations in patients with chronic obstructive pulmonary disease (COPD).
The drug was developed by GlaxoSmithKline (GSK) in collaboration with Theravance.
Breo Ellipta was approved by the US Food and Drug Administration (FDA) in May 2013 for the treatment of airflow obstruction in patients with COPD. Health Canada also approved the drug for the treatment of COPD patients in July 2013.
The drug received approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) for the treatment of bronchial asthma in September 2013.
It has received a positive response from the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) for the treatment of COPD patients in September 2013. The final approval from EMA is expected to be received by the end of 2013.
Breo Ellipta is marketed under the trade name Relvar Ellipta in Europe and Japan.
Chronic obstructive pulmonary disease (COPD) and causes
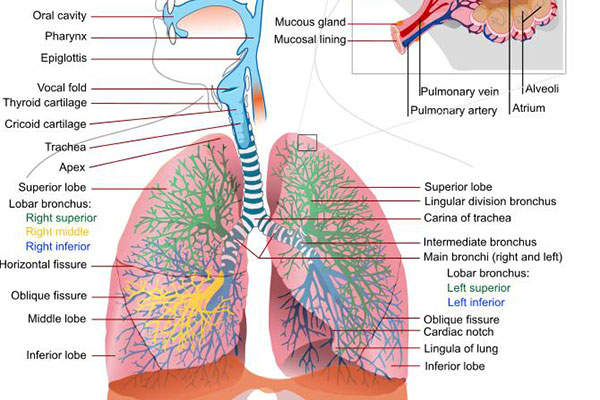
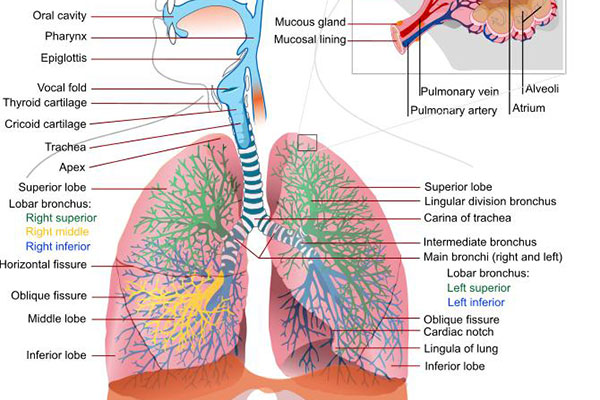
Chronic obstructive pulmonary disease (COPD) is a lung disease that restricts the flow of air to and from the lungs, causing shortness of breath.
The disease has two forms, namely chronic bronchitis, which involves a long-term cough with mucus, and emphysema, which involves destruction of the lungs.
Most of the patients affected by COPD have a blend of both forms of the disease. The main cause of the disease is tobacco smoking.
COPD is estimated to affect about 27 million people in the US, according to the National Heart, Lung and Blood Institute (NHLBI).
Breo Ellipta’s mechanism of action
Breo Ellipta contains two drug combinations, fluticasone furoate and vilanterol. Fluticasone furoate is an inhaled corticosteroid (ICS) and vilanterol is a long-acting beta2 agonist (LABA).
The drug helps breathing by reducing the risk of future exacerbations with a once-daily inhalation.
The drug is available in new dry powder inhaler (DPI) form, which contains 100µg of fluticasone furoate and 25µg of vilanterol.
Clinical trials of Breo Ellipta (fluticasone furoate and vilanterol)
The FDA approval for Breo Ellipta was based on 52 clinical pharmacology studies conducted on 1,406 patients and 11 clinical studies conducted on 7,851 patients with COPD. The clinical studies included four primary COPD studies, two six-month lung-function studies and two one-year replicate exacerbation studies.
GSK conducted a Phase I clinical trial on Breo Ellipta between December 2011 and March 2012. The randomised, open-label, crossover assignment enrolled 30 patients with COPD.
The primary outcome measure of the study was finding the pharmacokinetic parameters for fluticasone furoate (FF) for all study participants between pre-dose and 36 hours post-dose in each of the six treatment periods. The secondary outcome measure included finding the adverse events (AEs) for all participants in the study.
GSK conducted a Phase III clinical trial on Breo Ellipta between February 2011 and October 2011. It was also a randomised, double blind, and parallel assignment. The study enrolled 531 patients aged 40 years and above.
The primary outcome measure of the study was finding the change from baseline trough in 24-hour weighted-mean serial FEV1 on day 84. The secondary outcome measures included finding time to onset.
Commercialisation and marketing of Breo Ellipta in the US
Breo Ellipta was launched in the US market in 2013. The other medications approved for the treatment of the same indication include Tudorza Pressair developed by Forest Laboratories and Almirall, and Spiriva developed by Boehringer Ingelheim and Pfizer.
GSK entered into an agreement with Theravance for the development of and commercialisation of long-acting beta2 agonist (LABA) products in November 2002.
In March 2004, both the companies entered into a strategic alliance for development and commercialisation of different therapeutic areas, which gave an option for GSK to license exclusive development and commercialisation rights of the product candidates from certain of Theravance’s discovery programs.
GSK licensed MABA program of Theravance for the treatment of COPD in 2005. Theravance entered into an agreement with Elan Corporation in May 2013 as per which, Elan will pay $1bn royalty payment to Theravance for offering 21% participation interest in potential future royalty payments associated with four respiratory programs that Theravance partners with GSK.
Related content
Anoro Ellipta for Treatment of Chronic Obstructive Pulmonary Disease, US
Anoro ellipta (umeclidinium bromide / vilanterol) is a dry powder for inhalation, indicated for the treatment of airflow obstructions in patients suffering from Chronic Obstructive Pulmonary Disease (COPD) or emphysema.
Adempas (Riociguat) for Treatment of Pulmonary Hypertension, US
Adempas (riociguat) is indicated for the treatment of pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH).