Viekirax (ombitasvir / paritaprevir / ritonavir) and Exviera (dasabuvir) is indicated for the treatment of genotype 1 chronic hepatitis C virus (HCV) infection and compensated cirrhosis. The drug was developed by AbbVie in collaboration with Enanta Pharmaceuticals.
The new drug application (NDA) seeking approval for Viekirax was submitted to US Food and Drug Administration (FDA) in April 2014.
The FDA granted priority review designation for Viekirax for the treatment of GT1 chronic HCV infection in June 2014. The drug was also granted breakthrough therapy designation by the FDA.
AbbVie received approval from the FDA for Viekirax for the treatment of patients with chronic genotype 1 HCV infection, with or without ribavirin (RBV) in December 2014. The drug will be marketed under the brand name VIEKIRA PAK in the US.
The marketing authorisation application (MAA) for Viekirax and Exviera for the treatment of genotype 1 (GT1) and genotype 4 (GT4) chronic HCV infection was submitted to European Medicines Agency (EMA) in May 2014. The application is being reviewed under accelerated assessment designation.
AbbVie received a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the EMA, recommending the approval of Viekirax and Exviera, with or without RBV, for the treatment of GT1 and GT4 chronic HCV infection in November 2014.
The final decision on marketing approval of Viekirax and Exviera by the European Commission (EC) is expected to come in the first quarter of 2015. If approved, the drug will be available for marketing in 28 EU states, as well as in Iceland, Liechtenstein and Norway.
Chronic hepatitis C virus (HCV) infection
HCV infection is a contagious, long-term liver disease caused by the hepatitis C virus. The disease, in chronic stage, may lead to severe complications including cirrhosis or liver cancer. It has been classified into six distinct genotypes, of which genotype 1 is the most common type found in the US, and is the most difficult to treat.
Genotype 4 is commonly found in the Middle East and African countries, particularly in Egypt.
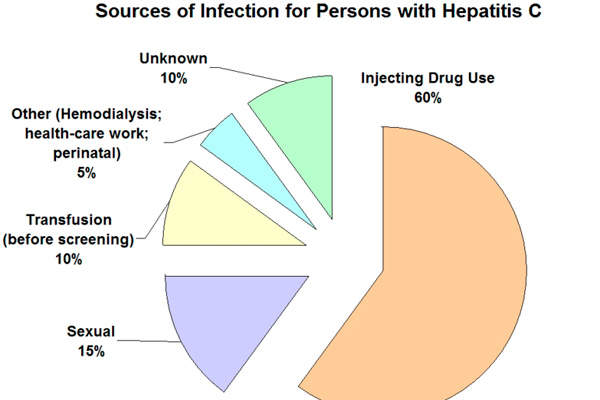
Approximately nine million people in Europe are estimated to have been infected with HCV. GT1 affects 60% of the world’s population, while GT4 is also becoming prevalent in European countries such as Italy, France, Greece and Spain.
Mechanism of action
Viekirax plus Exviera contains fixed-dose combinations of ombitasvir 25mg, paritaprevir 150mg, dasabuvir 250mg and ritonavir 100mg. The drug has a combination of three direct-acting antiviral drugs including ombitasvir, paritaprevir and dasabuvir, which act as NS5A inhibitor, NS3/4A protease inhibitor and non-nucleoside NS5B polymerase inhibitor respectively.
The drug can be used with or without the combination of ribavirin, an HIV-1 protease inhibitor that increases blood levels of paritaprevir.
Viekirax plus Exviera targets and inhibits specific HCV proteins and interrupts the viral replication process. The drug is available in the form of tablets for oral administration.
Viekirax clinical development programme details
The FDA approval, positive opinion and recommendation for approval by the CHMP was based on results obtained from a robust clinical development programme, which included six pivotal Phase III clinical trials known as Sapphire-I, Sapphire-II, Pearl-II, Pearl-III, Pearl-IV and Turquoise-II. The clinical programme enrolled more than 2,300 subjects with GT1 infection across 25 countries.
The FDA and CHMP also considered results from a Phase II study named Pearl-I, which enrolled GT4 subjects without cirrhosis, as well as those from Turquoise-I study, which enrolled GT4 patients co-infected with HIV-1, and Coral-I study, which enrolled liver transplant recipients with recurrent GT1 HCV infection.
Sapphire-I and Sapphire-II clinical trials
Incivek (telaprevir) is a protease inhibitor used for treating hepatitis C infections in liver patients.
Sapphire-I and Sapphire-II were placebo-controlled studies conducted on adult, non-cirrhotic patients with chronic genotype 1 (GT1) HCV infection.
Sapphire-I was a global, multi-centre, randomised, double-blind, placebo-controlled study conducted to evaluate safety and efficacy investigational treatment regimen with RBV. It enrolled 631 non-cirrhotic patients infected with GT1a and GT1b HCV, who were new to the therapy.
A total of 473 patients were randomised to receive AbbVie regimen with RBV for 12 weeks, and 158 were randomised to receive placebo for the initial 12 weeks. Patients further received open-label treatment with the AbbVie regimen with RBV for 12 weeks.
Sapphire-II was a global, multi-centre, randomised, double-blind, placebo-controlled study, which was conducted to evaluate safety and efficacy of investigational treatment regimen with RBV. The study enrolled 394 non-cirrhotic adult patients infected with GT1a and GT1b HCV infection, who previously failed treatment with pegylated interferon and RBV.
It enrolled 394 patients, of which 297 were randomised to receive AbbVie regimen with RBV for 12 weeks, and 97 patients were treated with placebo for an initial 12 weeks, and thereafter received open-label treatment with AbbVie regimen with RBV for 12 weeks.
Results of the Sapphire-I and Sapphire-II studies demonstrated that patients who received the AbbVie regimen with RBV for 12 weeks achieved sustained virologic response rates (SVR12) of 96.2% and 96.3% respectively after 12 weeks of post-treatment.
Pearl II and Pearl III clinical trials
Pearl-II was a multi-centre, randomised, open-label, controlled Phase III clinical study conducted to evaluate safety and efficacy of investigational treatment regimen, with and without RBV.
It enrolled 179 GT1b HCV-infected, treatment-experienced adult patients with no evidence of liver cirrhosis. Patients were divided into two arms, including 91 patients in the first arm, who were randomised to the regimen without RBV for 12 weeks, and 88 patients in the second arm, who were randomised to the regimen with RBV for 12 weeks.
The study results demonstrated that 100% of patients treated in the ribavirin-free arm achieved SVR12, while 97% of patients in the ribavirin-containing arm achieved SVR12.
Pearl-III was a multi-centre, randomised, double-blind, placebo-controlled clinical trial, which evaluated safety and efficacy of the treatment in non-cirrhotic, adult patients infected with GT1b HCV infection, and were new to treatment.
The study enrolled 419 patients with different demographics and characteristics randomised in two arms. The first treatment arm consisted of 209 patients treated with the regimen without RBV for 12 weeks, whereas the second treatment arm included 210 patients, who were randomised to the regimen with RBV for 12 weeks.
Patients in the first arm also received placebo in substitution for RBV.
The study met both primary and secondary endpoints. After 12 weeks of the study, 99% patients in arm one and 99.5% in arm two achieved SVR12.
Turquoise II clinical trials
Turquoise-II was the first Phase III study exclusively conducted in GT1 cirrhotic patients. The multi-centre, randomised, open-label, global, Phase-III clinical study was conducted to evaluate safety and efficacy of treatment regimen with RBV. It enrolled 380 adult GT1a and GT1b, treatment-naive and treatment-experienced patients with GT1 chronic HCV infection with compensated liver cirrhosis.
Patients were randomised in two treatment arms with 208 patients administered the regimen with ribavirin for 12 weeks in one arm, and 172 patients treated with the regimen with ribavirin for 24 weeks in the other.
The study achieved both primary and secondary end points. Patients treated with AbbVie’s regimen achieved SVR12 with 91.8% patients in the 12-week arm and 95.9% in the 24-week arm.