Farydak (panobinostat, previously known as LBH589) is a histone deacetylase (HDAC) inhibitor indicated for the treatment of patients with multiple myeloma. The drug was discovered and developed by Novartis.
In February 2015, Novartis received approval from US Food and Drug Administration (FDA) for Farydak in combination with bortezomib and dexamethasone for the treatment of multiple myeloma patients who previously received at least two regimens including bortezomib and an immunomodulatory (IMiD) agent.
Multiple myeloma
Multiple myeloma, also known as plasma cell myeloma, is the second most common blood cancer, which emanates in the plasma cells in bone marrow. It results in plasma cells producing antibodies and also restrains production of normal blood cells.
It is estimated that one to five in every 100,000 people worldwide are affected by multiple myeloma. The National Cancer Institute estimates that approximately 21,700 people in the US are diagnosed with multiple myeloma every year, of which 10,710 die from the disease.
Farydak’s mechanism of action
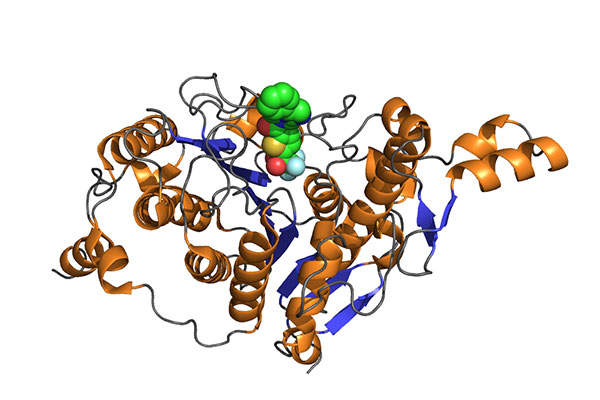
Farydak contains a histone deacetylase (HDAC) inhibitor that restrains the enzymatic activity of HDACs at nanomolar concentrations. The drug shows more cytotoxicity towards tumour cells compared to normal cells. It is available in 20mg dosed capsules for oral administration.
Clinical trials on Farydak
Kyprolis (carfilzomib), developed and manufactured by Onyx Pharmaceuticals, is a proteasome inhibitor indicated for the treatment of the patients with multiple myeloma.
Marketing approval for Farydak by the FDA was based on results obtained from a Phase III clinical trial. The randomised, double blind, placebo-controlled, multicentre clinical study enrolled 768 patients with relapsed multiple myeloma that received one to three prior lines of therapy.
Patients were administered with either bortezomib, dexamethasone in addition to Farydak 20mg, or placebo plus bortezomib and dexamethasone. The treatment was continued for a maximum of 16 cycles (48 weeks).
The primary endpoint of the study was progression-free survival (PFS), which was assessed through modified European Bone Marrow Transplant Group (EBMT) criteria.
Results of the study demonstrated the median PFS in the Farydak, bortezomib, dexamethasone treatment arm was 12 months, whereas it was 8.1 months in the placebo plus bortezomib and dexamethasone arm.
Farydak’s safety and efficacy were evaluated in a prespecified subgroup analysis of 193 patients who received prior treatment with both bortezomib and an immunomodulatory agent and a median of two prior therapies.
Results of the study showed the median PFS was 10.6 months in the patients treated with Farydak, bortezomib, and dexamethasone and 5.8 months in the placebo plus bortezomib and dexamethasone arm.
Adverse effects associated with the use of Farydak in the clinical study included diarrhoea, fatigue, nausea, peripheral oedema, decreased appetite, pyrexia and vomiting.
Marketing commentary
Farydak will be dispensed by Diplomat Pharmacy in the US. Novartis selected Biologics as limited distribution network partner of Farydak in March 2015.
Other medications available for the treatment of multiple myeloma include Pomalyst (Pomalidomide) manufactured by Celgene Corporation, and Kyprolis (carfilzomib) developed by Onyx Pharmaceuticals and Ligand Pharmaceuticals.