The Nuwiq injection is the first recombinant anti-haemophilic factor (blood coagulation factor VIII) drug indicated for the treatment of patients with haemophilia A.
The drug was discovered and developed by Octapharma USA, a subsidiary of global human protein products manufacturer Octapharma.
Nuwiq was approved by US Food and Drug Administration (FDA) in September 2015, as an intravenous therapy to control bleeding episodes in adults and children with haemophilia A.
The approval also includes on-demand treatment and control of bleeding episodes, regular prophylaxis to reduce the frequency of the episodes and peri-operative management of bleeding.
The drug was first approved by the European Commission (EC) in August 2014 followed by other countries, including Australia, the UK, Germany, Sweden, Canada, Italy and Argentina.
Nuwiq was launched into the US market in January 2016.
Octapharma was granted approval for three NUWIQ® vial strengths including 2500, 3000 and 4000 International Units (IU). The already available strengths are 250, 500, 1000 and 2000 IU.
Haemophilia A and its impact
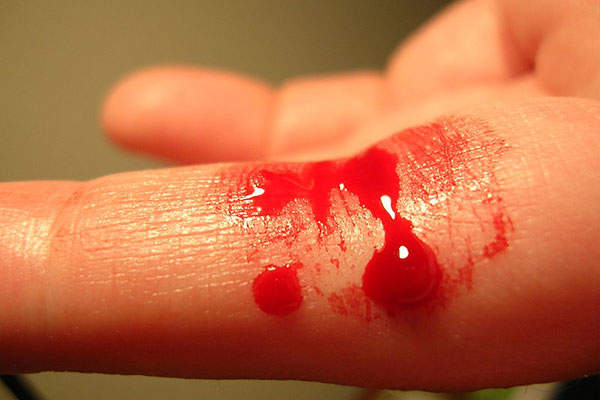
Haemophilia A is a genetic bleeding disorder caused by the deficiency of functional coagulation factor VIII, a clotting protein. It results in a prolonged clotting time leading to loss of blood during injuries.
People with haemophilia A bleed longer than average people. The frequency and severity of bleeding depend on the amount of FVIII in the plasma.
According to the US Centers for Disease Control and Prevention, approximately 20,000 people in the US are living with haemophilia A.
Nuwiq’s mechanism of action
Nuwiq contains an active ingredient called B-domain deleted recombinant coagulation factor VIII (BDD-rFVIII), which temporarily replaces the missing clotting factor VIII and aids required haemostasis.
The drug is available in the form of a white sterile, non-pyrogenic, lyophilised powder for reconstitution in single-use vials.
Nuwiq is the only recombinant factor VIII available with the lowest diluent volume of 2.5ml.
Clinical trials on Nuwiq
Obizur is a recombinant DNA derived anti-haemophilic factor. It replaces the inhibited coagulation factor VIII with a recombinant porcine sequence factor VIII, needed for effective blood clotting.
Obizur Antihemophilic Factor (recombinant) for the Treatment of Acquired Haemophilia A, US
Obizur is a recombinant DNA derived anti-haemophilic factor. It replaces the inhibited coagulation factor VIII with a recombinant porcine sequence factor VIII, required for effective blood clotting.
FDA approval for Nuwiq comes from the results of a global clinical study programme that included several clinical trials conducted to determine the safety and efficacy of the drug.
The initial global study included an open-label, multi-centre clinical trial, which enrolled 22 previously treated patients (PTPs). Results of the study showed the drug demonstrated a mean half-life of 17.1 hours using a one-stage clotting assay in adults.
The second set of studies included three multi-centre, open-label, prospective clinical studies conducted on PTPs with severe haemophilia A.
A total of 135 patients, including 74 adults, three adolescents and 58 paediatric patients, were treated with Nuwiq with a total of 16,134 infusions over 15,950 exposure days.
In another study enrolling 32 adults, the overall prophylactic efficacy of the drug for spontaneous bleeds rated excellent or good in 92% of patients. In a study conducted on 59 children, the same was rated excellent or good in 97% of patients.
Compared to haemophilia A patients receiving on-demand treatment, patients treated with Nuwiq prophylaxis witnessed a reduction in annualised bleeding rates of 96% for adults and 93% for children. Treatment of breakthrough bleeds during Nuwiq prophylaxis was rated as excellent or good.
On-demand treatment with Nuwiq was recorded as excellent or good in 931 of 986 (94%) bleeds, while the efficacy of prophylaxis in surgical was rated excellent or good in 32 of 33 (97%) procedures.
Adverse events noted during the studies were paraesthesia, headache, injection site inflammation, injection site pain, back pain, vertigo and dry mouth.