GLS-5700 is a synthetic vaccine being developed by Inovio Pharmaceuticals and GeneOne Life Sciences to prevent and treat infections caused by the Zika virus.
Inovio announced in early-2016 that a pre-clinical study conducted in mice and non-human primates generated significant immune responses. This demonstrates the vaccine’s capability in preventing viral infection.
After receiving approval by the US Food and Drug Administration (FDA) on 26 July, the company began phase I human trials for GLS-5700. The study also received approval from the health products and food branch of Health Canada on the same date.
Zika virus infection causes and symptoms
Zika virus infection is caused through the bite of an infected Aedes species mosquito. The virus can also be transmitted from mother to feotus during pregnancy, or from person to person through sexual intercourse with an infected partner. It is also likely spread through blood transfusion.
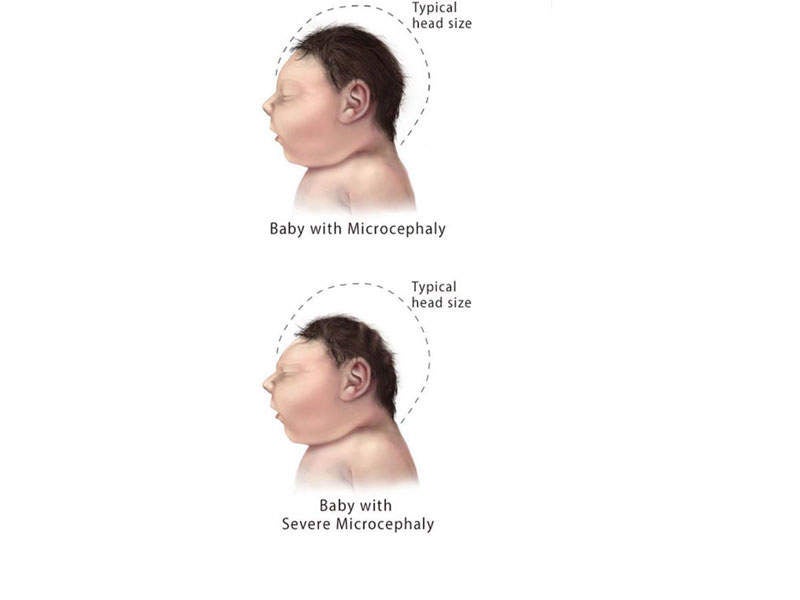
When transmitted from mother to a foetus, the infection causes a birth defect known as microcephaly. This can cause problems with eyesight, hearing, and impaired growth.
The Zika viral infection is also known to cause Guillain-Barré syndrome in adults, a condition causing limb muscle weakness and total paralysis in some cases. The virus has also been known to cause other neurological abnormalities.
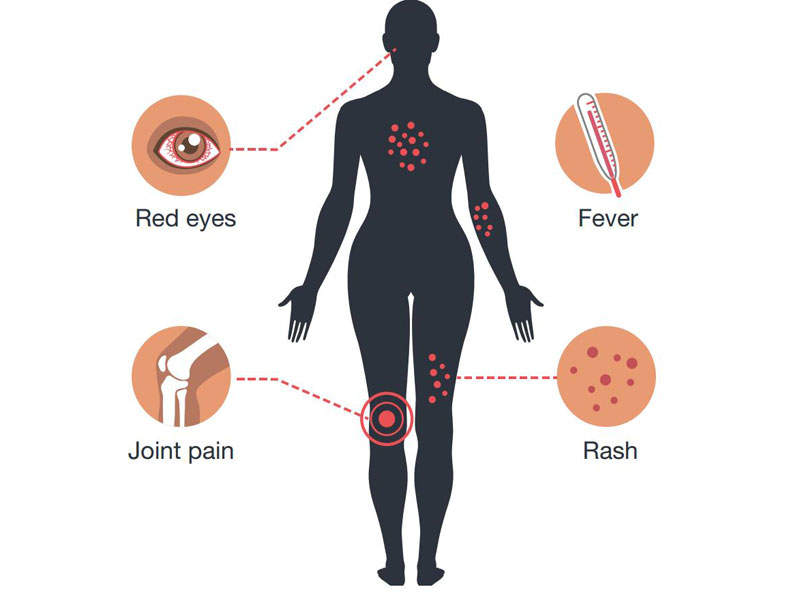
The infection does not usually show symptoms but may cause mild fever, rashes, joint and muscle pain, headaches, and red eyes. No treatment is currently available.
GLS-5700 DNA vaccine’s mechanism of action
GLS-5700 is a DNA plasmid vaccine being developed by Inovio using their proprietary SynCon Immunotherapy technology. While traditional vaccines are created using a single virus strain, Inovio’s technology uses multiple strains of the targeted infectious disease.
A gene sequence similar to that of the antigen strain is then created synthetically. The technology helps protect against new and emergent strains.
GLS-5700 induces protective antibodies and therapeutic T-cell responses in the body against multiple strains of Zika virus.
Clinical trials on GLS-5700
Inovio has been conducting pre-clinical studies and phase I clinical trials to evaluate the safety, efficacy, tolerability, and immunogenicity of the vaccine.
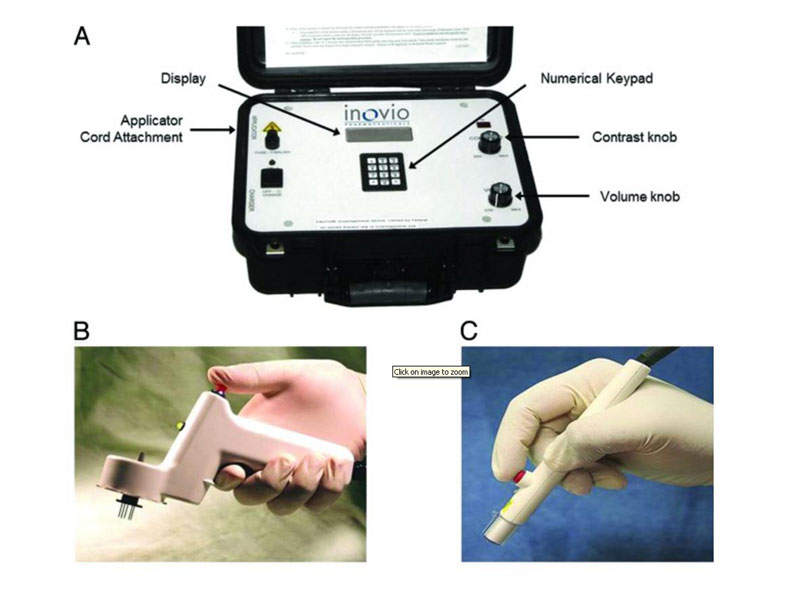
In pre-clinical studies, mice and monkeys were vaccinated with GLS-5700 using Inovio’s CELLECTRA electroporation delivery device either intramuscularly or intradermally. The device has been observed to maximise antigen expression and immune response in studies conducted earlier by Inovio.
Results from the pre-clinical studies, analysed by the T-cell ELISPOT assay, showed the vaccine induced robust immune responses in all the vaccinated animals.
Inovio initiated a phase I, open-label, dose-ranging study called Zika 001 in July. The trial is being conducted on 40 healthy adults to examine the safety and efficacy of the vaccine in human subjects. It is expected to be completed by November 2017.
A second phase I study on healthy adults was initiated in August. This placebo-controlled, double-blind trial is being conducted to elucidate the safety, efficacy, tolerability, and immunogenicity of the vaccine. The trial will enrol 160 subjects. Of these, 80 will receive placebo. The trial is expected to be completed by May 2018.
The exploratory end point of the trial will be the difference in the rate of Zika infection in the subjects administered with the vaccine when compared to placebo.