Radicava™ (edaravone) is a neuroprotective agent indicated for the treatment of amyotrophic lateral sclerosis (ALS).
The drug was discovered and developed by Mitsubishi Tanabe Pharma America, a pharmaceutical company based in the US.
The company submitted a new drug application (NDA) to the US Food and Drug Administration (FDA) for Radicava™’s approval in June 2016, which was accepted for review in August 2016.
Approved by the FDA in May 2017, the drug became one of the first intravenous infusions to be approved for the treatment of a rare disease in more than 20 years. The first drug approved by the FDA for ALS treatment was Rilutek® (riluzole) in December 1995.
Radicava™ was also approved in Japan and South Korea in June 2015 and December 2015, respectively. In the same year, the drug obtained orphan designation from the European Commission (EC) and the FDA.
ALS disease details
Also known as Lou Gehrig’s disease or motor neurone disease (MND), ALS is a rare progressive neurodegenerative disorder characterised by dysfunction of the nerves that control voluntary muscles movement including chewing, walking, breathing, and talking.
The condition causes muscles to become weak and ultimately leads to paralysis. The disease initially causes mild symptoms such as muscle weakness or muscle atrophy. Most patients die from respiratory failure within three to five years.
The Centers for Disease Control and Prevention estimates that between 12,000 and 15,000 people in the US are affected by ALS, while between 5,000 and 6,000 people a year are newly diagnosed with the disease in the country.
Radicava’s mechanism of action
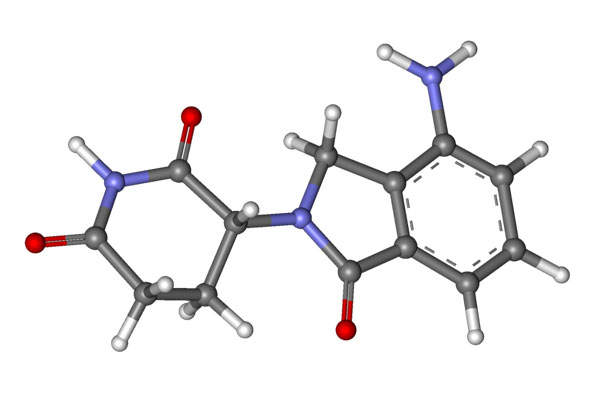
Radicava™ contains edaravone, a nootropic and neuroprotective agent used for neurological recovery. It is thought to act as a free radical scavenger and prevent oxidative stress damage to neurones.
The drug is available as a 60mg dose, which can be administered by intravenous infusion. It is initially infused daily for 14 successive days, followed by a two-week, drug-free period. It is then infused daily for ten days within a 14-day period, followed by a two-week drug-free period.
Clinical trials on Radicava
The Radicava™ clinical development programme included multiple phase III clinical trials. FDA approval was based on a pivotal phase III clinical trial known as MCI186-19, which was a double-blind, placebo-controlled study that evaluated the efficacy and safety of Radicava™.
The study enrolled 137 patients with ALS, who were randomised in 1:1 ratio to receive Radicava™ 60mg intravenously for 60 minutes or placebo for six-months. The primary endpoint of the study was a change in the ALS functional rating scale-revised (ALSFRS-R) score from baseline to six months. The revised ALSFRS was used to measure disease status and levels of disability in patients with amyotrophic lateral sclerosis.
Results of the study demonstrated that patients treated with Radicava™ exhibited less decline in physical function by 33% compared to placebo, at week 24. The study showed that patients treated with Radicava™ witnessed less decline in physical function by 2.49 ALSFRS-R points compared those in the placebo group.
The most common adverse reactions found in patients treated with Radicava were bruising, gait disturbance, and headache.
Radicava™’s efficacy with long-term patients and the effect on survival is yet to be evaluated.
Marketing commentary on MT Pharma America
Based in Jersey City, Mitsubishi Tanabe Pharma America is a wholly-owned subsidiary of Japanese company Mitsubishi Tanabe Pharma Corporation (MTPC).
MT Pharma America was established by MTPC to commercialise the group’s approved pharmaceutical products in the US. The company plans to launch Radicava™ in the US market in August 2017.