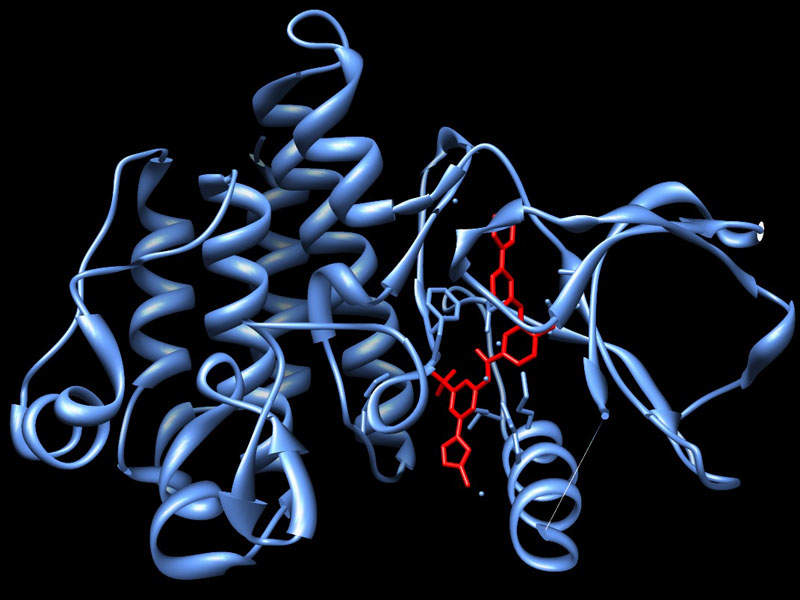
Nerlynx™ (neratinib) is a tyrosine kinase inhibitor indicated for the treatment of adult patients with early stage HER2-overexpressed or amplified breast cancer.
The drug was developed by US-based company Puma Biotech, which received US Food and Drug Administration (FDA) approval for Nerlynx™ following adjuvant trastuzumab-based therapy in July 2017.
The European Commission (EC) granted approval to a marketing authorisation application (MAA) for Nerlynx™ in August 2018.
HER2-positive breast cancer causes and symptoms
Breast cancer symptoms include lumps in the breast, a change of shape, dimpling of the skin, fluid coming from the nipple, or a red scaly patch of skin.
HER2-positive breast cancer constitutes between 20% and 25% of all types of breast cancer cases. It is more aggressive than other types and spreads faster.
More than 3.1 million breast cancer survivors are estimated to be present in the US.
Nerlynx’s mechanism of action
Nerlynx™ contains a tyrosine kinase inhibitor (TKI) that binds to Epidermal Growth Factor Receptor 2 (HER2) and HER 4. The drug reduces downstream growth, promoting signal transduction pathways and HER2 expressing carcinoma cell lines.
The drug is available in the form of 240mg tablets for oral administration.
Clinical trials on Nerlynx
Puma Biotechnology enrolled patients for a Phase III clinical trial known as NALA on Nerlynx™ in July 2017. The study enrolled 600 patients with third-line HER2-positive metastatic breast cancer. The patients were randomised to receive PB272 plus Xeloda or Tykerb plus Xeloda. The trial was conducted in North America, Europe and the Asia-Pacific (APAC).
The co-primary endpoints of the study included progression-free survival (PFS) and overall survival (OS).
The FDA and EC approvals for Nerlynx™ were based on results obtained from a Phase III clinical trial known as ExteNET trial, which was a multi-centre, randomised, double-blind, placebo-controlled study. It enrolled 2,840 women with early-stage HER2-positive breast cancer that had two years of completing adjuvant trastuzumab. The patients were randomised to receive either Nerlynx™ or placebo for one year.
Results of the study demonstrated that patients treated with Nerlynx™ showed invasive disease-free survival (iDFS) of 94.2% compared to iDFS of 91.9% in the placebo group after two years.
The most common adverse reactions found in patients treated with Nerlynx™ were diarrhoea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite and muscle spasms, as well as dyspepsia, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) increase, nail disorders, dry skin, abdominal distention, weight loss and urinary tract infections. The most common adverse reaction that led to the discontinuation of the clinical trial was diarrhoea.
Marketing commentary on Puma Biotechnology
Puma Biotechnology is a biopharmaceutical company focused on in-licensing innovative drug candidates for cancer care and developing them further for commercialisation. Most of the company’s drug candidates are under development for the treatment of HER2-positive breast cancer.
The medicines currently available in the market for the treatment of HER2 positive breast cancers include Kadcyla developed by Genentech and Tykerb developed by GlaxoSmithKline.