South Korean-based biotherapeutics firm SillaJen and French biotechnology firm Transgene have commenced patient enrolment in Europe for the ongoing Phase III PHOCUS clinical trial of Pexa-Vec to treat advanced liver cancer or hepatocellular carcinoma (HCC).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Pexa-Vec employs SillaJen's selective oncolytic vaccinia engineering (SOLVE) platform with a vaccinia strain backbone that targets cancer cells.
The product candidate is engineered to delete the thymidine kinase (TK) gene and express the immunogenic GM-CSF protein.
Initiated in January last year, the multinational, randomised, open-label PHOCUS trial is currently being carried out in North America, Asia, Australia and Europe, with a target enrolment of 600 patients.
Designed to be conducted at around 140 sites, the trial will include patients who have failed locoregional therapies and are eligible for treatment with sorafenib (Nexavar).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSorafenib is an approved systemic treatment for advanced HCC.
SillaJen chief executive officer (CEO) Eun Sang Moon said: "We are pleased with the progress in our PHOCUS trial and are happy to report that we are now enrolling patients in 11 countries across the globe.
"This trial, which is actively enrolling patients with HCC, is being conducted at some of the most highly regarded institutions for cancer in the world, and we are grateful to be working with such an exemplary team of physicians."
The first European patient recruitment into the trial triggers a $4m funding by Transgene to SillaJen.
The trial's primary objective is the measure of overall survival, while the secondary objectives include safety, assessments for tumour responses as measured by the time to progression, progression-free survival, overall response rate and disease control rate.
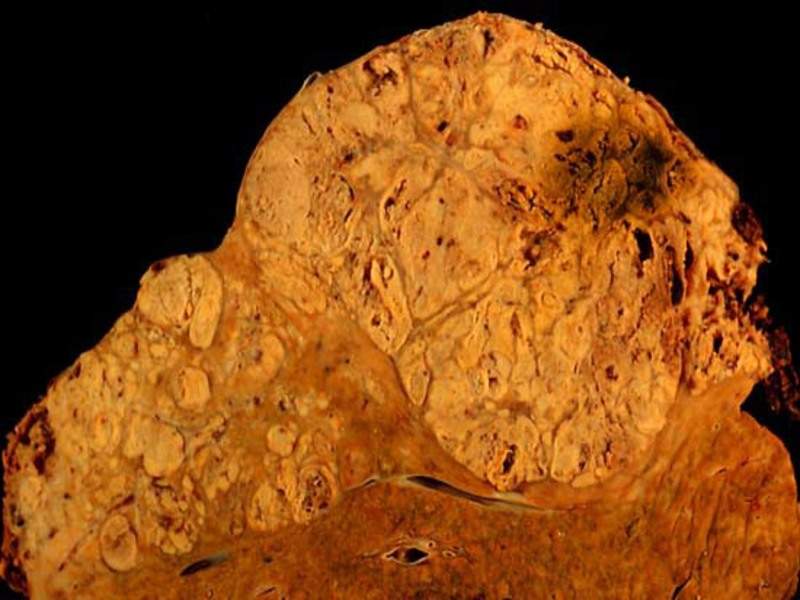
Image: Hepatocellular carcinoma. Photo: courtesy of Ed Uthman/Wikipedia.





